Abstract
Phox2a is a vertebrate homeodomain transcription factor that is involved in the specification of the autonomic nervous system. We have isolated the 5′ regulatory region of the human Phox2agene and studied the transcriptional mechanisms underlying its expression. We first identified the minimal gene promoter by means of molecular and functional criteria and demonstrated that its activity relies on a degenerate TATA box and a canonical Sp1 site. We then concentrated on the region immediately upstream of the promoter and found that it stimulates transcription in a neurospecific manner because its deletion caused a substantial decline in reporter gene expression only in neuronal cells. This DNA region contains a putative binding site for homeodomain transcription factors, and its mutation severely affects the transcriptional activity of the entire 5′ regulatory region, thus indicating that this site is necessary for the expression of Phox2a in this cellular context. The use of the electrophoretic mobility shift assay showed that Phox2b/PMX2b is capable of specifically interacting with this site, and cotransfection experiments demonstrated that it is capable of transactivating the human Phox2a promoter. Many data obtained from knock-out mice support the hypothesis that Phox2a acts downstream of Phox2b during the development of most of the autonomic nervous system. We have provided the first molecular evidence that Phox2b can regulate the expression of Phox2a by directly binding to its 5′ regulatory region.
Keywords: human, transcription factors, autonomic nervous system, transcriptional regulation, homeoproteins, promoter
One of the central issues in modern neurobiology is to understand how neuronal cell fate is determined. The neural crest is the cell type that has provided the most detailed information concerning the mechanisms controlling the fate of multipotential neural progenitors (Anderson, 1997; Francis, 1999), and, over the past few years, many aspects of the developmental program culminating in the formation of the autonomic nervous system have been elucidated. These studies have indicated the central role of two highly homologous transcription factors, Phox2a and Phox2b (also known as ARIX/PMX2A and PMX2b, respectively).
Phox2a and Phox2b are homeodomain proteins that are precociously and very similarly expressed in the three peripheral divisions of the autonomic nervous system (Valarché et al., 1993; Tiveron et al., 1996; Pattyn et al., 1997). They are also expressed in all of the adrenergic and noradrenergic neurons of the brainstem, in some cranial sensory ganglia that participate in the autonomic reflexes, and in a subset of cranial motor neurons. All of the components of the autonomic nervous system fail to properly develop in Phox2b knock-out (KO) mice, whereas Phox2a null mutants show a less severe phenotype involving the agenesis of the locus coeruleus and the atrophy of cranial sensory ganglia (Morin et al., 1997; Hirsch et al., 1998; Pattyn et al., 1999). These and other in vivo studies have also demonstrated that, with the exception of the cranial sensory ganglia, Phox2a acts downstream of Phox2b and Mash1, a basic helix-loop-helix transcription factor that has been shown to promote competence for neurogenesis in neural crest progenitors (Lo et al., 1997, 1998; Stanke et al., 1999). Together with Phox2b, Phox2a seems to act upstream of Ret [a subunit of the glia-derived neurotrophic factor receptor required for the survival of aggregating sympathoblasts (Durbec et al., 1996) and migrating enteric neuroblasts (Young et al., 1998)], and dopamine-β-hydroxylase, the last enzyme in the noradrenaline synthesis pathway (Zellmer et al., 1995; Goridis and Brunet, 1999).
Although these data suggest that Phox2a is probably involved in both the specification of autonomic phenotypes and the terminal differentiation of sympathetic neurons, most of its functional role is still enigmatic: it is expressed in all of the ganglionic neurons of the peripheral autonomic nervous system but appears to be necessary for the appropriate development of only a subset of sympathetic and parasympathetic ganglia (Morin et al., 1997). A better understanding of the transcriptional mechanisms underlying the expression of Phox2a and the molecular definition of the regulatory circuits that link it with Mash1 and Phox2b may provide new insights into its role. To this end, we cloned the 5′ regulatory region of the human Phox2a gene and characterized some of its molecular and functional properties.
MATERIALS AND METHODS
RNA preparation and Northern blot analysis. The RNAs were prepared and the Northern blot analysis performed as described inFlora et al. (2000). The RNAs were hybridized with a mouseApaI–ApaI cDNA probe spanning from nucleotide 687 to nucleotide 1218 of the published sequence, corresponding to the C terminus and part of the 3′ UTR of Phox2a (Valarché et al., 1993) (GenBank accession number X75014).
Molecular cloning of the human Phox2a 5′-flanking region. A human Phox2a cDNA probe was obtained from the SY5Y human neuroblastoma cell line by means of RT-PCR using primers designed on the first exon. The upper primer was 5′-CGGGCGCTCAGAGCTGGCCTCG-3′ [nucleotide (nt) +51/+72; see Fig. 2B]; the lower primer was 5′-CATGGCCGCCACGCACGAGTCG-3′ (nt +219/+198 on the lower strand; see Fig. 2B). The expected 168 bp fragment obtained was completely sequenced on both strands and used to screen a commercial human genomic library (Clontech, Palo Alto, CA) from normal peripheral blood leukocytes, cloned in the λ vector EMBL3 SP6/T7. After three rounds of screening, we isolated six different clones, one of which was analyzed in detail, by means of restriction analysis and the sequencing of both strands.
Fig. 2.
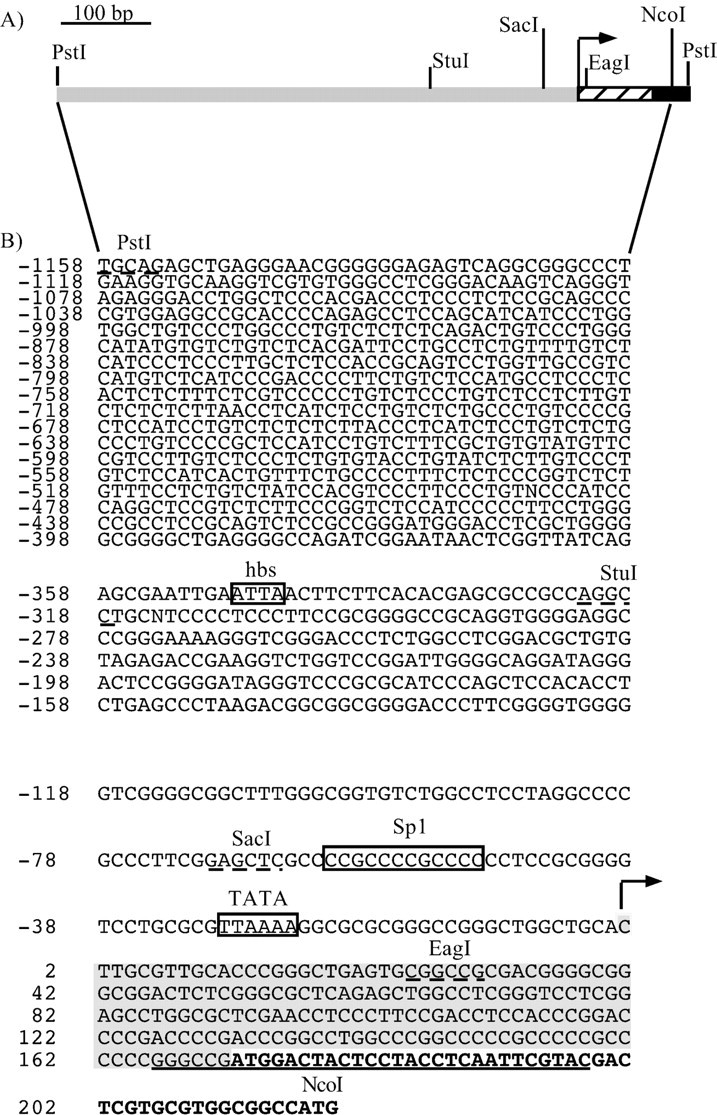
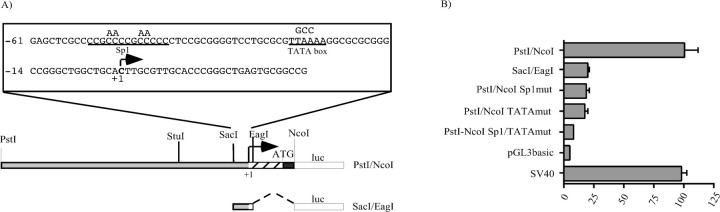
Structural features of the 5′-flanking region of the human Phox2a gene. A, Schematic representation of the PstI–PstI region showing the relevant restriction sites. The striped boxindicates the DNA sequence specifying the 5′ UTR of the mRNA; theblack region corresponds to the first part of the coding sequence. The arrow indicates the transcription start site. B, Sequence of thePstI–NcoI fragment. The positions are numbered from the transcription start site (indicated by thearrow), as defined by primer extension. The nucleotides in bold correspond to the human Phox2a coding sequence; the shaded region indicates the DNA sequence specifying the 5′ UTR of the mRNA, and the underlined sequencecorresponds to the primer used in the primer extension experiments. The putative TATA box and DNA binding sites for Sp1 and homeodomain transcription factors (hbs, homeodomain binding site) are boxed. The relevant restriction sites are indicated, and their sequences are underlined.
Primer extension. Total RNA from SY5Y cells expressing and HeLa cells not expressing Phox2a mRNA (see Fig. 1) was used to perform primer extension experiments.
Fig. 1.
Northern blot analysis of the expression of the human Phox2a gene in different human cell lines. A mouseApaI–ApaI cDNA probe, corresponding to the C terminus and part of the 3′ UTR of Phox2a, was hybridized to 10 μg of total RNA purified from the indicated cell lines.Top, The cDNA identified a unique transcript in neuroblastoma cell lines. Bottom, The hybridization signals obtained using human cDNA for the ribosomal RNA 18S after stripping of the Phox2a probe.
The experiments were performed using a32P-labeled oligonucleotide (5′-GTACGAATTGAGGTAGGAGTAGTCCATCGGCCC-3′) complementary to the human Phox2a ATG surrounding sequence [ nt +166/+198; underlinedin Fig. 2B]. Briefly, an amount of oligonucleotide corresponding to 5 × 105 cpm was coprecipitated with 20 μg of total RNA and elongated with 200 U Superscript II (BRL-Life Technologies, Gaithersburg, MD) at 50°C for 90 min. Yeast RNA was also elongated as a negative control. The reaction products were run on a 6% polyacrylamide denaturing gel. A DNA sequence was run in parallel as a molecular marker.
Construction of the human Phox2a promoter reporter plasmids. All of the reporter constructs were obtained by subcloning fragments of the human Phox2a 5′-flanking region into the pGL3basic plasmid (Promega, Madison, WI) upstream of the Firefly luciferase gene. A PstI–PstI genomic fragment (see Fig.2A) was first subcloned in pBluescript II KS and completely sequenced on both strands. From this construct we isolated the SacI–NcoI region containing the first 48 nt of the human Phox2a coding sequence and the entire 5′ UTR (see Fig.2A) and subcloned this fragment in pGL3basic to obtain the SacI/NcoI construct in which the sequence encoding the first 16 amino acids of Phox2a was fused in frame with the coding sequence of the luciferase enzyme (see Fig.4A). To generate the PstI/NcoI construct, we digested the PstI–PstI fragment cloned in pBluescript II KS with SacI, the sites of which were located in the Phox2a 5′-flanking sequence (see Fig. 2) and in the polylinker of the vector. The resulting 1140bpSacI–SacI fragment was gel-purified and subcloned in the SacI site of theSacI/NcoI construct in the proper orientation.PstI/EagI was created by digesting thePstI–PstI fragment cloned in pBluescript II KS with EagI, the sites of which were located in the Phox2a 5′-flanking sequence (see Fig. 2) and in the polylinker of the vector; the resulting fragment was blunt ended and subcloned in theSmaI site of pGL3basic, upstream of the luciferase 5′ UTR (see Fig. 4A). StuI/NcoI was obtained by digesting PstI/NcoI withPstI and StuI, blunting, and religating.SacI/EagI was derived from thePstI/EagI construct using the same cloning strategy. SV40 has already been described (Flora et al., 2000). The pRL-TK plasmid was purchased from Promega.
Fig. 4.
Functional mapping and characterization of the Phox2a minimal promoter. A, Schematic representation of the PstI/NcoI andSacI/EagI constructs. Thestriped box indicates the DNA sequence specifying the 5′ UTR of the mRNA; the black region corresponds to the first part of the coding sequence, and the ATG marks the start codon. The arrow indicates the transcription start site. The relevant restriction sites are shown. TheFirefly luciferase reporter gene (luc) is represented as an open box. The sequence in the enlargement above corresponds to theSacI–EagI region. The putative TATA box and Sp1 site are indicated, with the nucleotide substitutions introduced by recombinant PCR shown above. pGL3basic and SV40, which contain no promoter or the viral promoter SV40, were used as negative or positive controls, respectively. B, Luciferase assays. SY5Y cells were transiently transfected with the constructs indicated on the left, and the luciferase assays were performed 48 hr later. The bars represent the transcriptional activity of the constructs given as a percentage of thePstI/NcoI construct. The data represent the means ± SE (error bars) of at least three independent experiments performed in duplicate.
To introduce point mutations into the putative TATA box, Sp1 site, and homeoprotein binding site (hbs) (see Fig. 2, hbs), we exploited the recombinant PCR technique (Higuchi, 1990) using thePstI/NcoI construct as a template. Briefly, two complementary oligonucleotides bearing mutations in the core sequence of the putative DNA binding site of interest were used independently in separate amplification reactions, together with external primers corresponding to plasmid sequences. The two amplification products were then mixed together, denatured, and subjected to 10 extension cycles. The resulting DNA was subsequently amplified with the external primers, digested with the appropriate restriction enzymes, subcloned in pGL3basic, and sequenced. The Pfx thermostable DNA polymerase (BRL-Life Technologies) was used in all of the amplification steps. The derived constructs were namedPstI/NcoI TATAmut,PstI/NcoI Sp1mut, PstI/NcoI Sp1/TATAmut (when both mutations were present simultaneously), andPstI/NcoI hbsmut. The oligonucleotides (corresponding to nucleotides −65/−43) used to mutate the Sp1 site were Sp1mut UP (5′-CGCCCCGAACCGAACCCCTCCGC-3′) as upper primer and Sp1 mut LOW (5′-GGAGGGGTTCGGTTCGGGGCGAGCT-3′) as lower primer.
The oligonucleotides (corresponding to nucleotides −40/−14) used to mutate the TATA box were TATA mut UP (5′-GGTCCTGCGCGTGCCAAGGCGCGCGG-3′) as upper primer and TATA mut LOW (5′-CCGCGCGCCTTGGCACGCGCAGGACC-3′) as lower primer.
The oligonucleotides used to mutate the hbs site were hbsmut UP (5′-TCAGAGCGAATTGACGGAACTTCTTCA-3′, corresponding to nucleotides −363/−337) as upper primer and hbsmut LOW (5′-GTGTGAAGAAGTTCCGTCAATTCGCTCTGA-3′, corresponding to nucleotides −334/−363) as lower primer.
PRS1 has been described by Yang et al. (1998).
The letters in bold indicate the mutated nucleotides. All of the oligonucleotides listed above were purchased from Life Technologies.
The external oligonucleotides used in the PCR reactions were the Promega RV3 and GL2 primers designed on the pGL3basic vector.
The Phox2b expression construct was obtained by subcloning mouse Phox2b cDNA (Pattyn et al., 1997) in the XhoI and EcoRI sites of pCDNA3 (Invitrogen, Groningen, The Netherlands).
Transient transfections and luciferase assays. The cells were transiently transfected by means of lipofection using 2 × 105 SY5Y cells or 4 × 104 HeLa cells. Briefly, the cells were plated onto a well of a six-multiwell tissue culture plate (Falcon) the day before the transfection. Forty femtomoles of the construct of interest were mixed with an equimolar amount of pRL-TK and added to 1 μl of FUGENE 6 (Hoffman-La Roche, Basel, Switzerland). The DNA-lipid mixture was added to the cells and incubated for 36 hr, when luciferase activity was measured. The pRL-TK plasmid expresses theRenilla luciferase reporter gene under the control of the thymidine kinase minimal promoter and was cotransfected in each sample to normalize transfection efficiency. In the cotransfection experiments with Phox2b, 150 fmol of the Phox2b expression construct was added to the reporter and pRL-TK plasmids and incubated with 2 μl of FUGENE 6. Firefly and Renilla luciferase activities were detected using the Dual Luciferase Reporter Assay System (Promega). All of the transfections were performed in duplicate, and each construct was tested in at least three independent experiments using different batches of plasmid preparation. All of the plasmids were purified using Qiagen (Hilden, Germany) columns. The transient transfection data were analyzed as described previously (Battaglioli et al., 1998).
In vitro DNA–protein interaction analyses. The nuclear extracts were obtained as described in Terzano et al. (2000). To prepare the probes for footprinting experiments, theStuI–NcoI fragment was subcloned in pGEM-5Z (Promega) and digested with NcoI to label the bottom strand or with SalI to label the top strand. After filling in with32P-α-dCTP, the DNA was digested withSalI or NcoI. The excised fragments were run on a 5% nondenaturing polyacrylamide gel and gel purified. In each footprinting reaction, 2 fmol of the probe (∼20,000–30,000 cpm) were coincubated with 45 μg of nuclear extracts. Each reaction was performed in 50 μl containing 25 μl 2× binding buffer (1× binding buffer: 20 mm HEPES, pH 7.9, 2 mm MgCl2, 4% Ficoll, 0.5 mm DTT), 2 μg double-stranded poly(dI-dC), and 100 mm final salt concentration (NaCl + KCl). The nuclear extracts were preincubated on ice for 45 min and, after the addition of the probe, further incubated for 60 min. DNaseI was diluted in 1× binding buffer, 100 mm KCl, and 20 mm MgCl2 and used at a concentration 0.1–0.6 U/μg DNA in the samples with BSA, and 5–15 U/μg DNA in the presence of the extract. The DNaseI treatment was performed by adding 5 μl of DNaseI dilution to each reaction followed by incubation at room temperature for 150 sec. Digestion was stopped by adding 55 μl of Stop buffer 2× (1×: 100 mmNaCl, 10 mm Tris-HCl, pH 8, 10 mm EDTA, pH 8, 0.5% SDS, 5 μg/ml proteinase K). After 30′ of incubation at 50°C, the DNA was phenol–chloroform extracted, ethanol precipitated, resuspended in denaturing loading dye (NaOH 0.1 m/formamide 1:2; 0.01% xylene cyanol), and loaded on a denaturing 6% polyacrylamide gel. A sequence was run in parallel to the reactions as a molecular weight marker.
All of the oligonucleotides used in the electrophoretic mobility shift assay (EMSA) experiments were labeled by fill-in and purified on a G25 Sephadex column (Boehringer Mannheim, Mannheim, Germany). With the Sp1 oligonucleotide, we used the footprint binding buffer, whereas the binding conditions for the hbs oligo were 12.5 mm HEPES, pH 7.9, 4 mm Tris-HCl, pH 7.9, 60 mm KCl, 1 mm EDTA, 4% Ficoll, and 1 mm DTT. Samples containing 10 μl of 2× binding buffer, 2 μg of double-stranded poly(dI-dC), 2 μg nuclear extract, and H2O to 20 μl were preincubated on ice for 20 min, and then 1 fmol of probe (corresponding to 10,000–20,000 cpm) was added. After a 40 min incubation, the reactions were loaded on a nondenaturing 5% polyacrylamide gel and run in Tris-Borate-EDTA 0.5× at a constant voltage of 150 V. Competition and supershift experiments were performed by adding the specific oligonucleotide or antibody to the reactions during the preincubation time. We used 0.1 and 1 pmol of cold oligonucleotides for the competition experiments. The oligonucleotides used in the EMSA experiments were the following: Phox2a/Sp1 (5′-CATGGAGCTCGCCCCGCCCCGCCCCCCTCCGC-3′, nucleotides −71 to −47); Phox2a/Sp1mut (5′-CATGGAGCTCGCCCCGAACCGAACCCCTCCGC-3′); hbs (5′-TCAGAGCGAATTGATTAAACTTCTTCA-3′, nucleotides −363–337); hbsmut is described above. The Sp1 and EGR oligonucleotides have been described previously (Terzano et al., 2000). All of the oligonucleotides were purchased from Life Technologies, and all of the antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
RESULTS
Detection of Phox2a transcript in human neuroblastoma cell lines
Neuroblastomas are pediatric tumors that are thought to arise from migratory cells of the embryonal neural crest; several clonal cell lines have been stabilized and have most of the features of the autonomic neurons, such as the ability to express ganglionic-type nicotinic receptors (Gotti et al., 1997).
To demonstrate that the neuroblastoma cell lines were capable of expressing Phox2a mRNA, we performed a Northern blot analysis. A unique transcript of ∼1.9 kb was observed in only the two human neuroblastoma cell lines, SY5Y and SK-N-BE, whereas no signal was detectable in HeLa and MOLT4 cells, two non-neuronal human cell lines of epithelial and lymphoid origin, respectively (Fig.1).
A very intense band was detected in all four lanes when the same blot was rehybridized with a probe for the 18S ribosomal RNA (Fig.1).
Structural and functional characterization of the human Phox2a 5′ regulatory region
To isolate the 5′-flanking region of the Phox2a gene, we screened a human genomic library with a probe corresponding to part of the first exon. We identified six different clones, all of which contained a 1411 bp PstI–PstI fragment that included most of the first exon (from nucleotide +1 to nucleotide +253) (Valarché et al., 1993) and 1158 bp of the upstream region of the human Phox2a gene (Fig. 2). Primer extension experiments were performed to map the initiation of transcription. A single band was observed in the SY5Y cells, whereas, as expected, no signal was detectable in HeLa cells and yeast RNAs (Fig. 3). Remarkably, the first transcribed nucleotide identified by these experiments corresponded to the first nucleotide of the cDNA sequence described by Johnson et al. (1996).
Fig. 3.
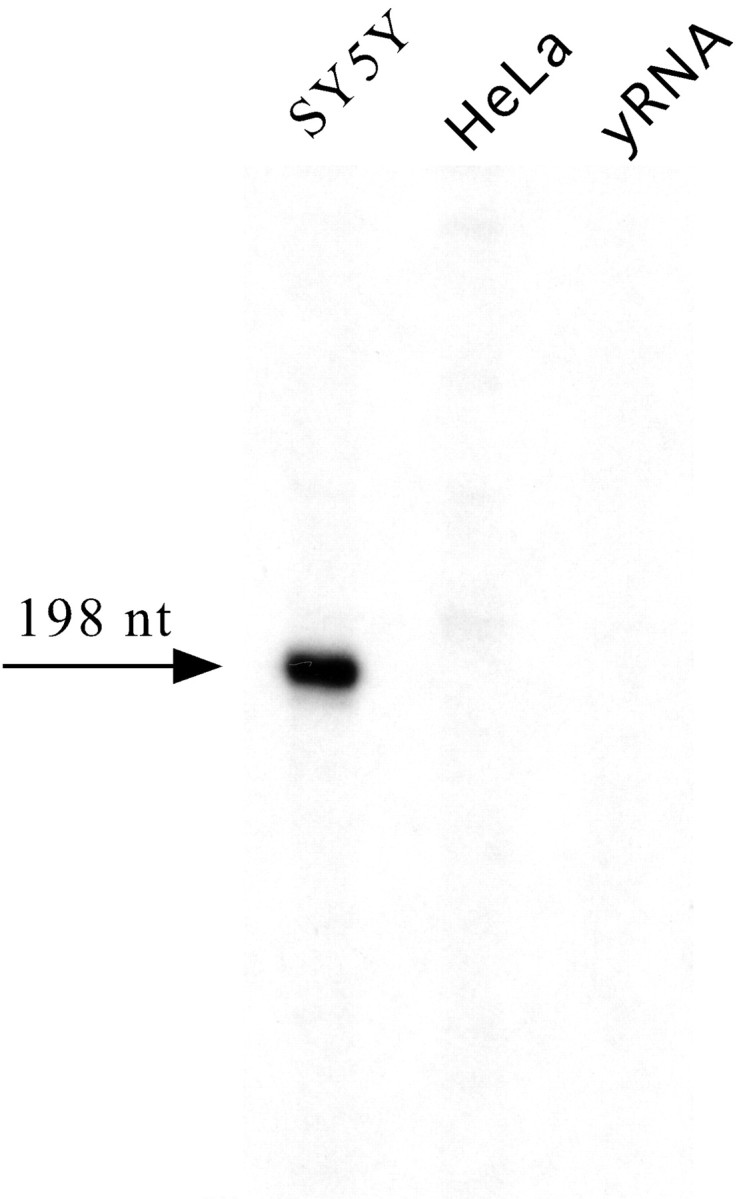
Primer extension mapping of the transcription start site of the human Phox2a gene. A 32P oligonucleotide complementary to the +166/+198 region of the humanPhox2a gene (see also Fig. 2B) was annealed to total RNA from the indicated cell lines and then extended. Yeast RNA (yRNA) was used as a negative control. The arrow indicates the only detected band, the length of which is also indicated (nt, nucleotide).
The presence of a unique initiation site suggested that transcription had to be driven by a promoter containing a TATA box or an Initiator element, or both. Consistently, computer-assisted analysis showed that a degenerate TATA box was present at the canonical location of −25 nucleotides from the transcription start site (Fig.2A). Moreover, a putative Sp1 binding site was identified a few nucleotides upstream of the TATA sequence (Fig.2A), thus supporting the hypothesis that the region we had isolated contained the core promoter and part of the regulatory region of the human Phox2a gene. To confirm this at a functional level, we cloned the PstI–NcoI fragment in front of the luciferase reporter gene and performed transient transfections in the SY5Y human neuroblastoma cell line. The construct showed as much as 25× the activity of the promoterless pGL3basic and worked slightly more than the plasmid SV40 that contains the SV40 viral promoter (Fig.4B).
Molecular and functional analysis of the human Phox2a minimal promoter
To demonstrate that the DNA region of the human Phox2agene (previously shown to contain a putative TATA box and a putative Sp1 site) could be engaged in interactions with nuclear proteins, we performed in vitro footprint experiments. A DNA fragment corresponding to the StuI–NcoI region was labeled on the bottom strand, incubated with either SY5Y or HeLa nuclear extracts, and treated with increasing amounts of DNaseI. As a negative control, the labeled DNA was digested with no nuclear extract and in the presence of BSA. These experiments showed that the DNA region encompassing the putative TATA box and Sp1 cis-acting elements was completely protected from digestion by both the SY5Y and HeLa extracts (Fig. 5). To demonstrate the functional contribution of these DNA elements to the activity of the Phox2a promoter, we introduced point mutations in thePstI/NcoI construct and performed transient transfection experiments in SY5Y (Fig. 4). The mutations in the Sp1 and TATA box sequences severely affected the activity of thePstI/NcoI construct: thePstI/NcoI Sp1mut andPstI/NcoI TATAmut constructs worked five times less than the parental plasmid, and the simultaneous mutation of the two sites completely abolished the transcriptional activity of the resulting plasmid PstI/NcoI Sp1/TATAmut, the activity of which was comparable with that of pGL3basic (Fig. 4). Conversely, the SacI–EagI construct containing only the Sp1 site, the TATA box, and the initiation of transcription still retained appreciable transcriptional activity (Fig. 4) (five times more than that of pGL3basic).
Fig. 5.
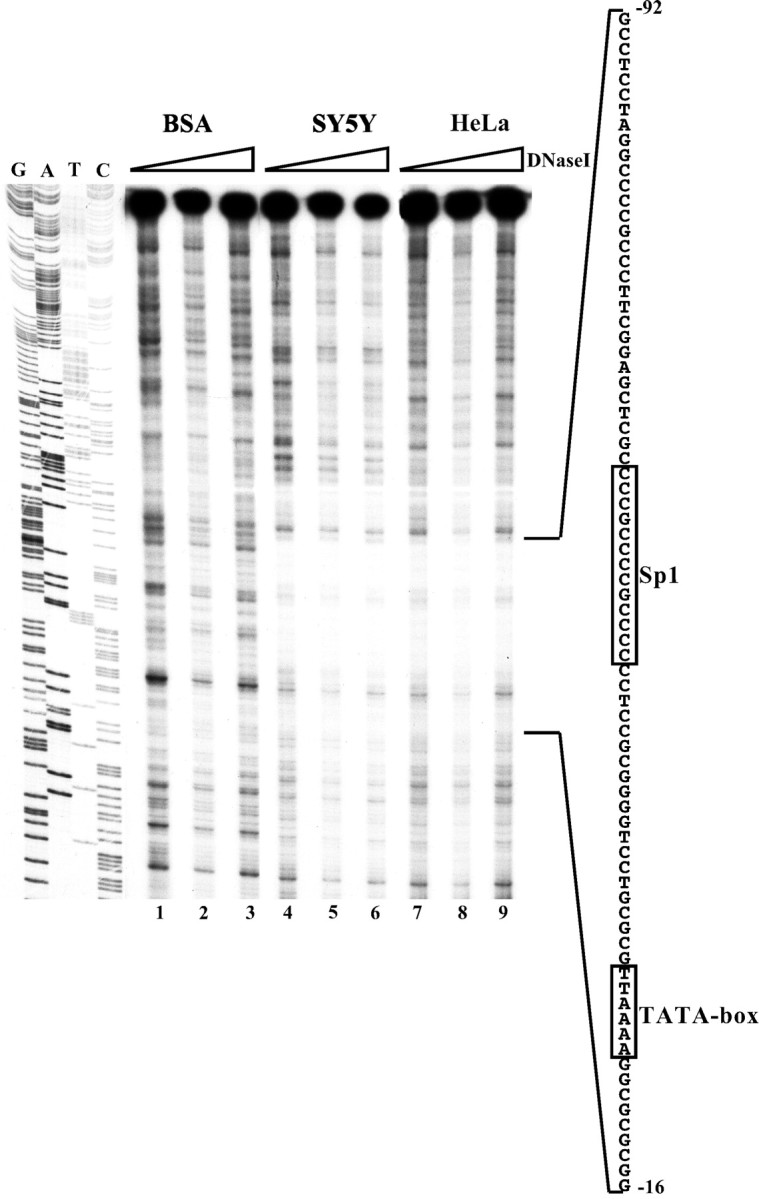
DNase footprinting of theStuI–NcoI region. TheStuI–NcoI fragment was labeled on the bottom strand and digested with increasing amounts of DNaseI in the presence of SY5Y (lanes 4–6) and HeLa (lanes 7–9) nuclear extracts. Digestions were also performed in the presence of BSA as a negative control (lanes 1–3). The enlargement on the right shows the sequence of the DNA region that was protected from digestion by both nuclear extracts. The TATA box and Sp1 site are boxed. On the left is a DNA sequence loaded in parallel as a molecular marker.
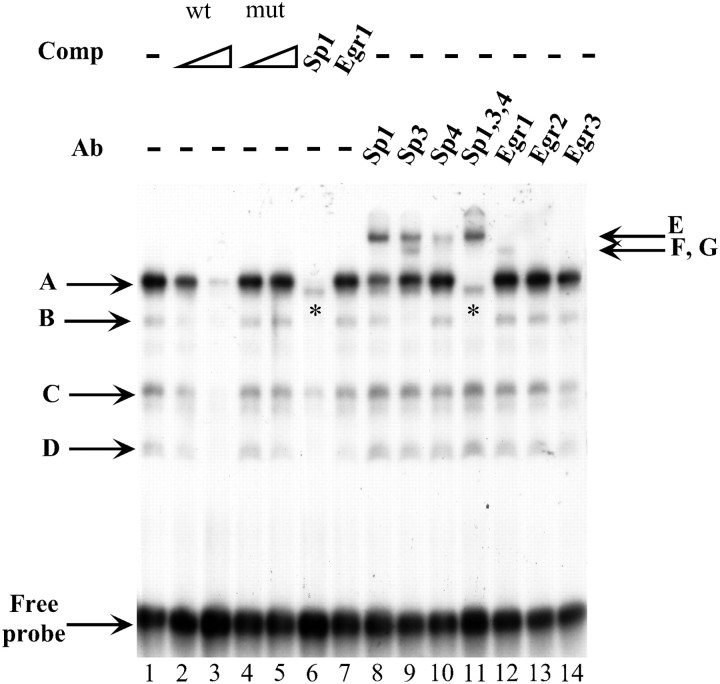
The DNA–protein interactions on the Sp1 consensus site were further characterized by EMSA. When a labeled oligonucleotide bearing the Phox2a Sp1 site was incubated with the SY5Y nuclear extract, four major retarded complexes were observed (Fig. 6,lane 1). All of these bands were competed by a molar excess of unlabeled Phox2a Sp1 oligonucleotide, whereas the addition of a mutated Phox2a Sp1 oligonucleotide did not have any effect (Fig. 6,lane 2-5). A canonical Sp1 binding site was capable of competing the A, B, and D bands but failed to inhibit a faint band immediately below the A band (Fig. 6, lane 6,asterisk) and only partially blocked the formation of the C complex. Competition with a canonical Egr1 oligonucleotide did not interfere at all with the formation of the four retarded bands (Fig. 6,lane 7). Supershift experiments were performed to confirm the involvement of Sp1 and to reveal the participation of other members of the Sp family. Antibodies against Sp1, Sp3, and Sp4 were all capable of inducing the formation of supershifted complexes (Fig. 6,lanes 8, 9, 10). In particular, band B was supershifted only by an anti-Sp3 antibody, and complex A was made of all three Sp proteins. The band immediately below the A band was not supershifted when antibodies against Sp1, Sp3, and Sp4 were added to the same sample (Fig. 6, lane 11, asterisk). The Egr1 antibody induced a very faint supershifted band (Fig. 6,lane 12), whereas the antibodies against Egr2 and Egr3 failed to shift any complex (Fig. 6, lanes 13,14).
Fig. 6.
Molecular characterization of the Sp1 site in the human Phox2a minimal promoter by EMSA. EMSAs were performed using a probe corresponding to a part of the Phox2a minimal promoter (from nucleotide −71 to −47) that contained the previously characterized Phox2a Sp1 site. On the left, the arrowsand corresponding letters (A,B, C, D) indicate four retarded complexes that formed on incubation of the labeled probe with the SY5Y nuclear extract (lane 1). Competition experiments were performed in the presence of a 100× or 1000× excess of the wild-type oligonucleotide (lanes 2–3) or in the presence of its mutated version in which the Sp1 site was mutagenized (lanes 4–5). Competition experiments were also performed using a 1000× excess of oligonucleotides bearing canonical sites for Sp1 and Egr1 (lanes 6–7). Supershift experiments were performed using the −71/−47 wild-type oligonucleotide together with the indicated antibodies (lanes 8–14). On the right, the arrows and corresponding letters (E,F, G) indicate the supershifted complexes. The asterisks mark a band that migrated immediately below the A complex (lanes 6 and11). The free probe is indicated.
Functional role of the DNA regions surrounding the minimal promoter
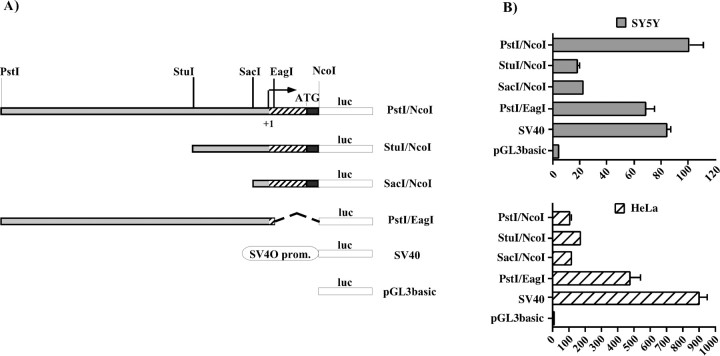
Comparison of the transcriptional activities of thePstI/NcoI and SacI/EagI constructs (Fig. 4B) clearly indicated that the DNA regions surrounding the minimal promoter played a fundamental role in the expression of Phox2a. A more detailed deletion analysis of thePstI–NcoI region in both SY5Y and HeLa cells revealed the presence of two functionally relevant subregions. The first corresponded to the PstI–StuI fragment, and its deletion produced a fivefold reduction in the expression of the reporter gene in SY5Y (Fig.7B, compare constructs Pst/NcoI and StuI/NcoI), whereas the same deletion led to a modest increase in transcriptional activity in HeLa cells. A further deletion of theStuI–SacI fragment had negligible effects on the expression of the luciferase gene in both cell lines (Fig.7B, compare constructs StuI/NcoI andSacI/NcoI). The PstI–StuI subregion therefore seemed to contain one or more elements that activated transcription only in neuroblastoma cells. The second subregion corresponded to most of the DNA region specifying the 5′ UTR of the mRNA. Its replacement with the 5′ UTR of the luciferase gene produced a fivefold increase in luciferase gene expression only in HeLa cells (Fig. 7B, compare the constructsPstI/NcoI and PstI/EagI). This suggested that the EagI–NcoI region contained one or more elements that negatively regulate the expression of the reporter gene in non-neuronal cells. In principle, these elements may act at either a transcriptional or post-transcriptional level, or both. In brief, thePstI–NcoI region seems to contain negative and positive cis-acting elements that restrict the activity of the human Phox2a promoter to neuroblastoma cells. In this study, we focused on the positive mechanisms that take place in neuronal cells.
Fig. 7.
Functional characterization of the 5′ regulatory region of the human Phox2a gene. A, Schematic representation of the reporter constructs used in the transient transfection experiments. The striped boxindicates the DNA sequence specifying the 5′ UTR of the mRNA; theblack region corresponds to the first part of the coding sequence, and the ATG marks the start codon. Thearrow indicates the transcription start site. The relevant restriction sites are shown. The Fireflyluciferase reporter gene (luc) is represented as anopen box. B, Luciferase assays. SY5Y and HeLa cells were transiently transfected with the constructs indicated on the left, and the luciferase assays were performed 48 hr later. The bars represent the transcriptional activity of the constructs given as a percentage of the activity of thePstI/NcoI construct. The data represent the means ± SE (error bars) of at least three independent experiments performed in duplicate.
Regulation of the human Phox2a 5′ regulatory region by homeoproteins
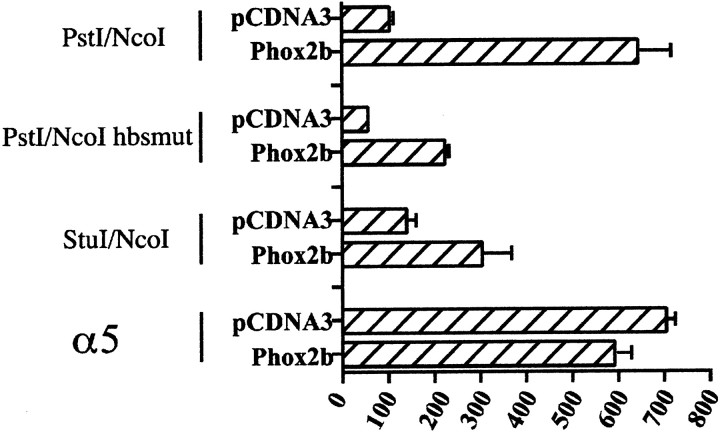
The PstI–StuI region contains a putative DNA binding site for homeoproteins (Fig. 2). To investigate whether this site could account for at least part of the positive transcriptional effects that this region exerted in neuroblastoma cells, we introduced three point mutations into the putative homeoprotein core sequence and transfected the resultingPstI/NcoI hbsmut construct into SY5Y cells. In comparison with its wild-type counterpart, this construct showed a dramatic decrease in the capability of driving the expression of the reporter gene, having a transcriptional activity that was similar to that of pGL3basic (Fig.8A). When these constructs were transfected into HeLa cells, the decrease in transcriptional activity was only 50% (Fig.9, compare thePstI/NcoI and PstI/NcoI hbsmut constructs when cotransfected with the empty pCDNA3 vector; and data not shown). In line with these results, an identical mutation of the hbs in the context of the PstI/EagI plasmid, the most active construct in HeLa cells, produced a similar decrease in transcriptional activity (data not shown).
Fig. 8.
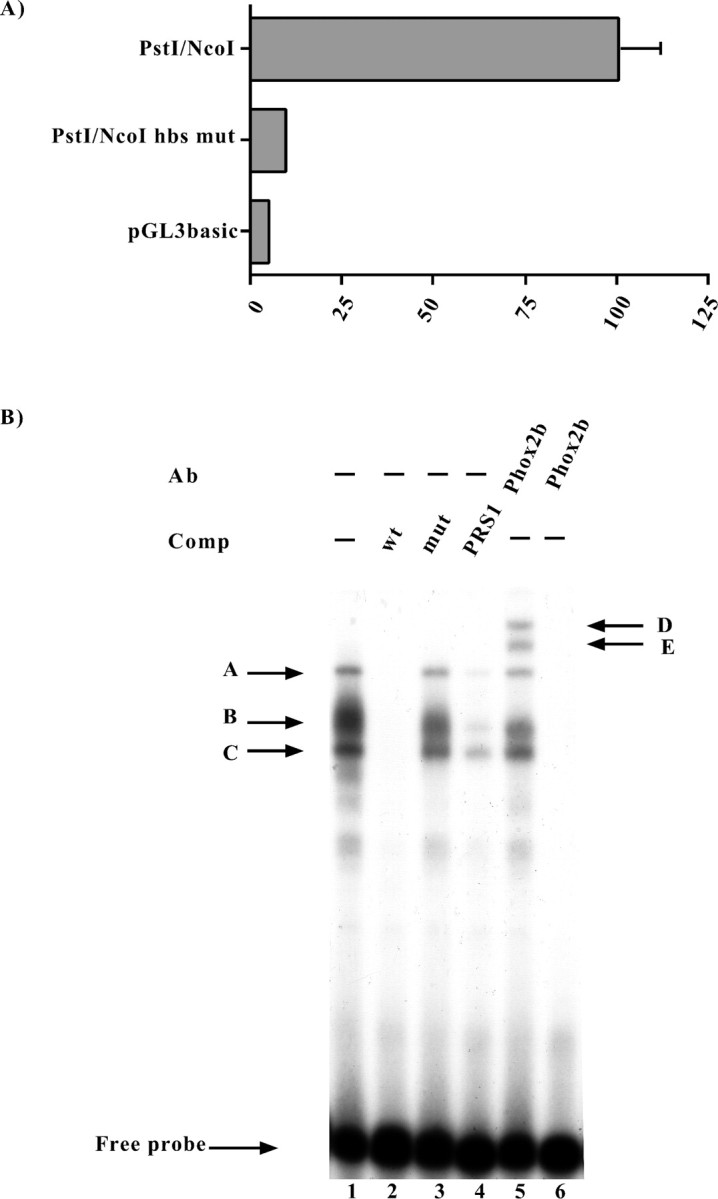
Functional and molecular characterization of the putative homeoprotein binding site (hbs).A, Luciferase assays. SY5Y cells were transiently transfected with the constructs indicated on the left, and the luciferase assays were performed 48 hr later. Thebars represent the transcriptional activity of the constructs given as the percentage of the activity of thePstI/NcoI construct. The data represent the means ± SE (error bars) of at least three independent experiments performed in duplicate. B, EMSAs were performed using a probe corresponding to the human Phox2a sequence between the nucleotides −363 and −337 (Fig. 2), which contained the putative homeoprotein binding site. On the left, thearrows and corresponding letters(A, B, C) indicate the retarded complexes that formed on incubation of the labeled probe with the SY5Y nuclear extract (lane 1). Competition experiments were performed in the presence of a 1000× excess of the wild-type oligonucleotide (lane 2) or in the presence of its mutated version in which the putative homeoprotein binding site was mutagenized (lane 3). The specificity of the DNA–protein interactions was also checked by means of competition with a cold oligonucleotide bearing the Phox2 site of thedopamine-β-hydroxylase gene (Yang et al., 1998) (lane 4). On the right, the arrows and corresponding letters(D, E) indicate the supershifted complexes obtained using an antibody directed against Phox2b (Pattyn et al., 1997) (lane 5). Lane 6, No nuclear extract.
Fig. 9.
Transactivation of the human Phox2a promoter by Phox2b. Luciferase assays were performed to measure the activity of the Phox2a promoter reporter constructs upon their cotransfection in HeLa cells with the pCDNA3 expression vector harboring the Phox2b cDNA or with the empty vector. The bars represent the transcriptional activity of the constructs (indicated on theleft) given as a percentage of the activity of thePstI/NcoI construct when cotransfected with the empty pCDNA3. The data represent the means ± SE (error bars) of at least three independent experiments performed in duplicate. α5 indicates the −1011/+180 construct described inFlora et al. (2000), which corresponds to part of the promoter region of the human α5 nicotinic subunit gene.
To demonstrate that the putative homeoprotein DNA binding site was capable of binding nuclear proteins, we performed gel-shift assays. When a labeled oligonucleotide corresponding to the Phox2a sequence that included the putative homeoprotein binding site was incubated with a SY5Y nuclear extract, three distinct retarded bands were observed, all of which were competed by a molar excess of cold oligonucleotide (Fig. 8B). On the contrary, a cold mutagenized oligonucleotide bearing the same mutations as those tested in the transfection experiments was unable to compete. Genetic evidence in mice indicates that Phox2a could be regulated by Phox2b, and so we performed competition experiments using a cold oligonucleotide containing the previously characterized Phox2 Responsive Sequence 1 (PRS1) of the dopamine-β-hydroxylase gene promoter (Yang et al., 1998). As shown in Figure 8B(lane 4), a molar excess of this oligonucleotide greatly reduced the formation of the three retarded bands. A more direct demonstration of the involvement of Phox2b in the observed gel-retardation pattern was provided by supershift experiments. When a previously characterized polyclonal antibody directed against Phox2b (Pattyn et al., 1997) was added to a reaction containing the SY5Y nuclear extract and the wild-type Phox2a hbs oligonucleotide, two additional ultra-retarded bands were observed (Fig.8B, lane 5).
Transactivation of the human Phox2a promoter by Phox2b
To obtain further functional evidence concerning the role of Phox2b in the expression of Phox2a, we performed cotransfection experiments in which a vector expressing Phox2b was introduced into HeLa cells together with different Phox2a reporter constructs (Fig. 9). A considerable transactivation was observed with the wild-type PstI/NcoI reporter construct, but, surprisingly, both the PstI/NcoI hbsmut and theStuI/NcoI constructs, which lacked the homeoprotein binding site, were partially transactivated in the presence of Phox2b. This partial response did not seem to be caused by an aspecific effect of Phox2b, because the regulatory region of the human α5 gene [construct −1011/+180 (Flora et al., 2000)], a cholinergic-nicotinic subunit that is normally expressed in HeLa cells, was not transactivated at all.
DISCUSSION
In this study, we started delineating the transcriptional mechanisms underlying the expression of the human Phox2agene in neuronal cells. Using molecular and functional criteria, we first identified the minimal promoter of the gene, the activity of which was found to rely on a degenerate TATA box and a canonical Sp1 site.
Sp1 is a ubiquitous protein that belongs to the superfamily of mammalian Sp/XKLF transcription factors, which consists of at least 16 different members (Philipsen and Suske, 1999). The most characterized subfamily is that of Sp1, Sp2, Sp3, and Sp4, which, with the exception of Sp2, preferentially bind to GC-rich elements identical to that identified in the human Phox2a promoter. Supershift experiments showed that all three transcription factors can bind to the Phox2a GC box. Because of their very similar affinity for the same DNA binding site, the significance of the coexpression of these three factors is not fully understood; however, it is worth noting that, under certain circumstances and as a result of competition, Sp3 and Sp4 can act as repressors of Sp1-activated transcription (Hagen et al., 1994; Majello et al., 1995; Kwon et al., 1999), and that the cell content of the three proteins can vary under different conditions, including proliferation and differentiation (Apt et al., 1996). At the level of the Phox2a promoter, this picture seems to be even more complicated because the Egr1 transcription factor appears to bind to the same region (although this needs to be confirmed by more directed experiments), and under certain circumstances, Egr1 can also interfere with Sp1-mediated transcription by competing for the same DNA site (Ebert and Wong, 1995). Both the competition and supershift data showed that, when the binding of all of the members of the Sp-family was prevented, a novel band appeared that could not be supershifted by the Egr1 antibody (data not shown). This band may simply reflect the binding of a nonspecific factor that binds the site with low affinity under these artificial conditions, or the binding of a non-Sp member of the Sp/XKLF superfamily. Taken together, these observations allow us to speculate that several levels of transcriptional control may exist at the human Phox2a promoter, most of which rely on the same DNA site. Experiments using HeLa cells gave very similar results (data not shown), except for Sp4, the interaction of which with the GC box in the Phox2a promoter was not detected (data not shown). This finding was expected because Sp4 is expressed much more in neuronal tissues (Philipsen and Suske, 1999), and, consistently, this distribution pattern is reproduced in neuroblastoma and HeLa cells (data not shown). However, the presence or less of the Sp4 binding did not apparently modify the function of the minimal promoter, which showed very similar transcriptional activity in both SY5Y and HeLa cells (data not shown). This suggested that the elements responsible for the neurospecific expression of the gene lie outside of this region.
We therefore studied the DNA regions immediately upstream and downstream of the core promoter and identified two subregions that have different effects in neuronal and non-neuronal cell lines. The first largely coincides with the part of the Phox2a gene specifying the 5′ UTR of the mRNA and contains one or more reducer elements that repress gene expression only in HeLa cells. The molecular characterization of this subregion will be described elsewhere, and we shall here concentrate on the upstream PstI–StuI region, which seemed to contain one (or more) positive neurospecific elements, because its deletion caused a considerable decline in luciferase expression only in SY5Y cells.
Computer-assisted analysis revealed that this DNA fragment contained a putative homeoprotein binding site. It has been shown that the homeoproteins involved in the development of the nervous system can sometimes regulate each other by creating complex transcriptional networks (Briscoe et al., 2000). The mutation of the Phox2a/hbs severely affected the transcriptional activity of thePstI/NcoI construct in SY5Y, thus indicating that this site is necessary for the expression of Phox2a in this cellular context. It has been shown previously in KO mice that, during the development of most of the autonomic nervous system, Phox2a seems to act downstream of the highly homologous homeodomain protein Phox2b (Morin et al., 1997; Pattyn et al., 1997, 1999; Dubreuil et al., 2000). The human Phox2b protein (also known as PMX2B) shares with its mouse ortholog an amino acid identity of 99% and an identical homeodomain, which is responsible for the interaction with DNA. The results of our EMSA and supershift experiments provide the first molecular evidence that Phox2b/PMX2b is capable of specifically interacting with the human Phox2a promoter. To show that it also plays a functional role inPhox2a expression, we performed cotransfection experiments in HeLa cells (which do not express any endogenous Phox2a or Phox2b) that clearly demonstrated the ability of Phox2b to transactivate the Phox2a promoter in a specific way, because it did not affect a control promoter. Phox2b still retained some transactivating activity on the Phox2a promoter even when its cognate DNA binding site was deleted or mutated. We hypothesize that Phox2b, regardless of DNA binding, can contact one or more components of the transcriptional apparatus that assembles at the human Phox2a core promoter, although such interactions are more stable and efficacious when Phox2b is also bound to DNA. This would be in line with the observation that Drosophila fushi tarazu is still capable of activating some of its target genes even in the absence of its DNA binding domain (Copeland et al., 1996). Taken together, these data suggest that the direct interaction with the cognate cis-acting element is to some extent dispensable for some homeodomain proteins.
In conclusion, we propose that the expression of the humanPhox2a gene primarily relies on a minimal promoter that potentially has several levels of regulation, perhaps also in response to external stimuli as suggested by the possible involvement of Egr1. The basal activity of this minimal promoter needs to be stimulated but also restricted to neuronal tissues, particularly to various structures of the autonomic nervous system. This seems to be achieved by combining positive and negative mechanisms that act in neuronal and non-neuronal contexts, respectively. We studied the positive mechanisms and found that Phox2b is one of the major players in the expression of Phox2a. This is the first study to provide information concerning the molecular events underlying the expression of a fundamental specifier of the human sympathetic neuron phenotype. This approach complements in vivo studies and will lead to a better understanding of how neuronal cell fate is established.
Note added in proof. The sequence of thePHOX2a gene promoter is available at EMBL Nucleotide Sequence Database under Accession no. AJ320270.
Footnotes
This work was supported in part by grants to Francesco Clementi from the Italian Ministry of University and Scientific and Technological Research (MURST Grant MM05152538) and from the European “Training and Mobility of Researchers” Program (Contract ERB4061PL97-0790). We thank Kevin Smart for his help in preparing this manuscript and Susanna Terzano for suggestions.
Correspondence should be addressed to Dr. Diego Fornasari, Department of Medical Pharmacology, University of Milan, Consigilo Nazionale delle Ricerche Cellular and Molecular Pharmacology Center, Via Vanvitelli 32, 20129 Milano, Italy. E-mail:D.Fornasari@csfic.mi.cnr.it.
REFERENCES
- 1.Anderson DJ. Cellular and molecular biology of neural crest cell lineage determination. Trends Genet. 1997;13:276–280. doi: 10.1016/s0168-9525(97)01187-6. [DOI] [PubMed] [Google Scholar]
- 2.Apt D, Watts RM, Suske G, Bernard Hu. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transformation correlate with the activation of the HPV-16 promoter. Virology. 1996;224:281–291. doi: 10.1006/viro.1996.0530. [DOI] [PubMed] [Google Scholar]
- 3.Battaglioli E, Gotti C, Terzano S, Flora A, Clementi F, Fornasari D. Expression and transcriptional regulation of the human alpha3 neuronal nicotinic receptor subunit in T lymphocyte cell lines. J Neurochem. 1998;71:1261–1270. doi: 10.1046/j.1471-4159.1998.71031261.x. [DOI] [PubMed] [Google Scholar]
- 4.Briscoe J, Pierani A, Jessel TM, Ericson J. A homeodomain code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 5.Copeland JWR, Nasiadka A, Dietrich BH, Krause HM. Patterning of the Drosophila embryo by a homeodomain-deleted Ftz polypeptide. Nature. 1996;379:162–165. doi: 10.1038/379162a0. [DOI] [PubMed] [Google Scholar]
- 6.Dubreuil V, Hirsch MR, Pattyn A, Brunet JF, Goridis C. The Phox2b transcription factor coordinately regulates neuronal cell cycle exit and identity. Development. 2000;127:5191–5201. doi: 10.1242/dev.127.23.5191. [DOI] [PubMed] [Google Scholar]
- 7.Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- 8.Ebert SN, Wong DL. Differential activation of the rat phenylethanolamine N-methyltransferase gene by Sp1 and Egr-1. J Biol Chem. 1995;270:17299–17305. doi: 10.1074/jbc.270.29.17299. [DOI] [PubMed] [Google Scholar]
- 9.Flora A, Schulz R, Benfante R, Battaglioli E, Terzano S, Clementi F, Fornasari D. Neuronal and extra-neuronal regulation of the expression of the human alpha5 nicotinic receptor subunit gene. J Neurochem. 2000;75:18–27. doi: 10.1046/j.1471-4159.2000.0750018.x. [DOI] [PubMed] [Google Scholar]
- 10.Francis NJ. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- 11.Goridis C, Brunet J. Transcriptional control of neurotransmitter phenotype. Curr Opin Neurobiol. 1999;9:47–53. doi: 10.1016/s0959-4388(99)80006-3. [DOI] [PubMed] [Google Scholar]
- 12.Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 13.Hagen G, Muller S, Beato M, Suske G. Sp-1 mediated transcriptional activation is repressed by Sp3. EMBO J. 1994;13:3843–3851. doi: 10.1002/j.1460-2075.1994.tb06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higuchi R. Recombinant PCR. In: Michael AI, editor. PCR protocols. Academic; San Diego: 1990. pp. 169–176. [Google Scholar]
- 15.Hirsch M, Tiveron M, Guillemot F, Brunet J, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KR, Smith L, Johnson DK, Rhodes J, Rinchik EM, Thayer M, Lewis EJ. Mapping of the ARIX homeodomain gene to mouse chromosome 7 and human chromosome 11q13. Genomics. 1996;33:527–531. doi: 10.1006/geno.1996.0230. [DOI] [PubMed] [Google Scholar]
- 17.Kwon HS, Kim MS, Edenberg HJ, Hur MW. Sp3 and Sp4 can repress transcription by competing with Sp1 for the core cis-elements on the human ADH5/FDH minimal promoter. J Biol Chem. 1999;274:20–28. doi: 10.1074/jbc.274.1.20. [DOI] [PubMed] [Google Scholar]
- 18.Lo L, Sommer L, Anderson DJ. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr Biol. 1997;7:440–450. doi: 10.1016/s0960-9822(06)00191-6. [DOI] [PubMed] [Google Scholar]
- 19.Lo L, Tiveron M, Anderson D. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;125:609–620. doi: 10.1242/dev.125.4.609. [DOI] [PubMed] [Google Scholar]
- 20.Majello B, De Luca P, Suske G, Lania L. Differential transcriptional regulation of c-myc promoter through the same DNA binding sites targeted by Sp1-like proteins. Oncogene. 1995;10:1841–1848. [PubMed] [Google Scholar]
- 21.Morin X, Cremer H, Hirsch M, Kapur RP, Goridis C, Brunet J. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997;18:411–423. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- 22.Pattyn A, Morin X, Cremer H, Goridis C, Brunet J. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- 23.Pattyn A, Morin X, Cremer H, Goridis C, Brunet J. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- 24.Philipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 1999;27:2991–3000. doi: 10.1093/nar/27.15.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanke M, Junghans D, Geissen M, Goridis C, Ernsberger U, Rohrer H. The Phox2 homeodomain proteins are sufficient to promote the development of sympathetic neurons. Development. 1999;126:4087–4094. doi: 10.1242/dev.126.18.4087. [DOI] [PubMed] [Google Scholar]
- 26.Terzano S, Flora A, Clementi F, Fornasari D. The minimal promoter of the human alpha3 nicotinic receptor subunit gene. Molecular and functional characterization. J Biol Chem. 2000;275:41495–41503. doi: 10.1074/jbc.M006197200. [DOI] [PubMed] [Google Scholar]
- 27.Tiveron M, Hirsch M, Brunet J. The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci. 1996;16:7649–7660. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valarché I, Tissier-Seta J, Hirsch M, Martinez S, Goridis C, Brunet J. The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development. 1993;119:881–896. doi: 10.1242/dev.119.3.881. [DOI] [PubMed] [Google Scholar]
- 29.Yang C, Kim H, Seo H, Kim C, Brunet J, Kim K. Paired-like homeodomain proteins, Phox2a and Phox2b are responsible for noradrenergic cell-specific transcription of the dopamine β-hydroxylase gene. J Neurochem. 1998;71:1813–1826. doi: 10.1046/j.1471-4159.1998.71051813.x. [DOI] [PubMed] [Google Scholar]
- 30.Young HM, Hearn CJ, Ciampoli D, Southwell BR, Brunet JF, Newgreen DF. A single rostro-caudal colonisation of the rodent intestine by enteric neuron precursors is revealed by the expression of Phox2b, Ret and p75, and by explants grown under the kidney capsule or in organ culture. Dev Biol. 1998;202:67–84. doi: 10.1006/dbio.1998.8987. [DOI] [PubMed] [Google Scholar]
- 31.Zellmer E, Zhang Z, Greco D, Rhodes J, Cassel S, Lewis EJ. A homeodomain protein selectively expressed in noradrenergic tissue regulates transcription of neurotransmitter biosynthetic genes. J Neurosci. 1995;15:8109–8120. doi: 10.1523/JNEUROSCI.15-12-08109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]