Fig. 5.
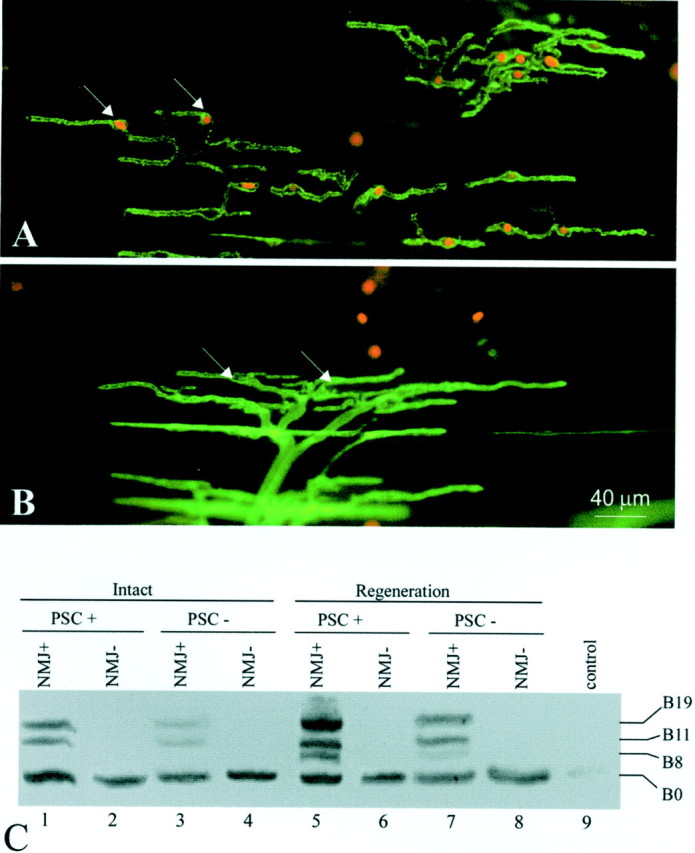
Agrin expression in perisynaptic Schwann cells at the neuromuscular junction. A, PSCs at the NMJ were selectively ablated in vivo by labeling the PSC membrane with mAb 2A12 and subsequent application of guinea pig complement, which forms membrane attack complexes and lyses cells. PSC lysis was revealed by ethidium homodimer-1, which enters cells with damaged membranes and stains their nuclei (in red,arrows). PSCs (in green) were revealed by labeling with FITC-conjugated secondary antibody to mAb 2A12. Over 80% of PSCs were ablated by this technique. B, A control muscle treated with complement alone did not show dead PSCs (arrows mark nuclei of living PSCs). On average, <5% of PSCs were ablated in the absence of mAb 2A12. PSCs in the control group were revealed with FITC-conjugated peanut agglutinin (ingreen). C, The acute effect of PSC ablation on agrin expression in normal and reinnervated muscles was examined by RT-PCR. Within the NMJ-rich region of intact CP muscle (PSC+), agrin expression typical of adult Schwann cells was observed (lane 1, compare with Fig.2A, Intact), whereas only the B0 isoform was found in the NMJ-poor region (NMJ−,lane 2), which consists mainly of muscle tissue. When PSCs were killed, active agrin bands (B11 and B19) became much weaker (lane 3). With PSCs intact, 2 weeks after axotomy, nerve regeneration induced the upregulation of B11/B19 and the appearance of B8 (lane 5), similar to Schwann cells along the regenerated sciatic nerve (lane 5, compare with Fig.2A). This upregulation of active isoforms was significantly weaker after PSC ablation (lane 7). NMJ-poor regions showed no response to PSC ablation either with or without denervation (lane 2 vs lane4, lane 6 vs lane 8).Lane 9, As a negative control, the lane showed no bands when no RNA samples were used for RT-PCR.
