Fig. 5.
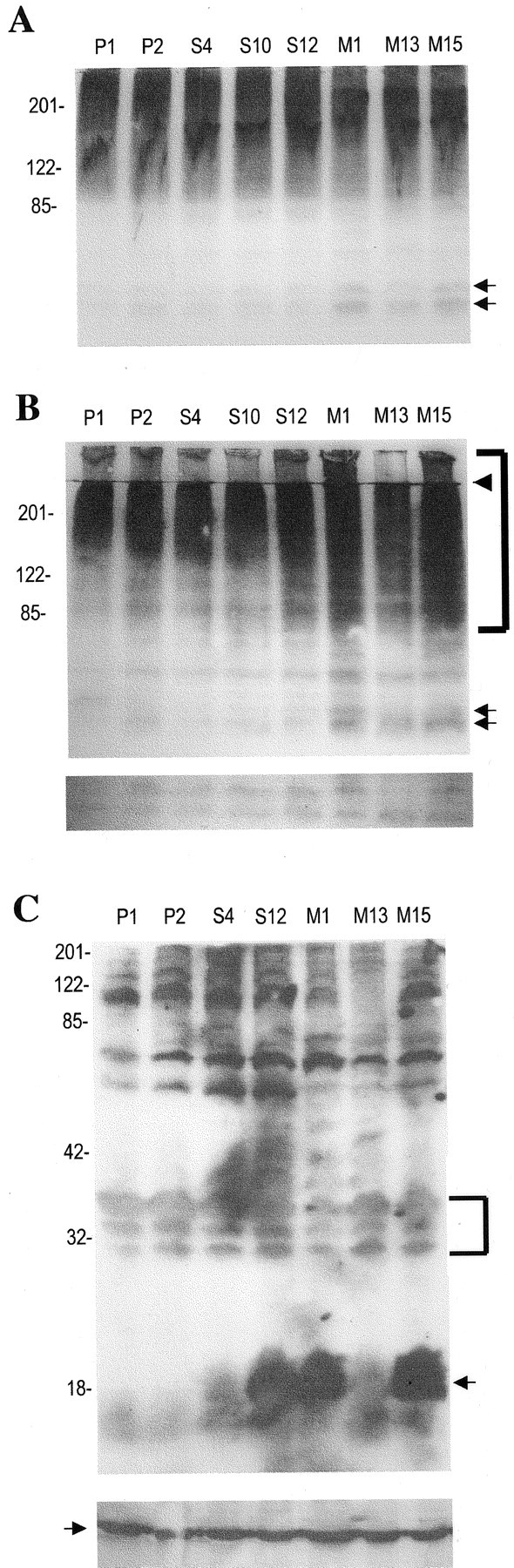
Increase of multi-ubiquitinated proteins in Triton-insoluble lysates of PC12 cells expressing A53T α-synuclein.A, Cell lysates (200 μg of protein) from various clonal lines were resolved on an 8% SDS-PAGE gel and immunoblotted with an anti-ubiquitin polyclonal antibody (1:1000; Dako). Thearrows indicate a doublet at 52–54 kDa that was selectively increased in M1 and M15 cell lysates. This blot represents one of two independent experiments, which yielded similar results.B, Triton-insoluble, sample buffer-soluble lysates (200 μg of protein) were resolved on an 8% SDS-PAGE gel and immunoblotted with an anti-ubiquitin polyclonal antibody (1:1000; Dako). Thearrows indicate bands at 52–54 that were more prominent in the M1 and M15 lines. Thebracket indicates higher molecular mass proteins, which were more prominent in the M1 and M15 lines. The arrowheadindicates the end of the stacking gel. The bottom panel is from a longer exposure (3 min vs 1 min) of the same blot, at the level of 30–35 kDa. The bands seen are presumably background bands, with similar intensity across the lanes. Equal protein loading was also verified by Ponceau S staining. This blot represents one of three independent experiments, which yielded similar results, except for the fact that an increase in polyubiquitinated proteins was inconsistently found for M13. C, Triton-insoluble, sample buffer-soluble lysates (200 μg of protein) from various clonal lines were resolved on a 12% SDS-PAGE gel and immunoblotted with anti-synuclein. The arrow indicates the 18 kDa α-synuclein band. Note that the exposure for this blot was at least 10 times longer than for the blots used to obtain similar band intensities from Triton-soluble lysates. The blot was intentionally overexposed to detect low abundance α-synuclein-specific bands in the upper portion of the blot. Such bands were not seen. The bands detected at the top portion of the blot are presumably nonspecific background bands, because they are also seen in the empty-vector control lysates. Thebracket indicates background bands at 30–35 kDa that are of similar intensity across the various samples. To ensure equal protein loading, the same samples were loaded on another gel and immunoblotted with an anti-actin monoclonal antibody (1:5000; Sigma) (bottom panel). The arrow in thebottom panel indicates the 44 kDa actin band.
