Fig. 3.
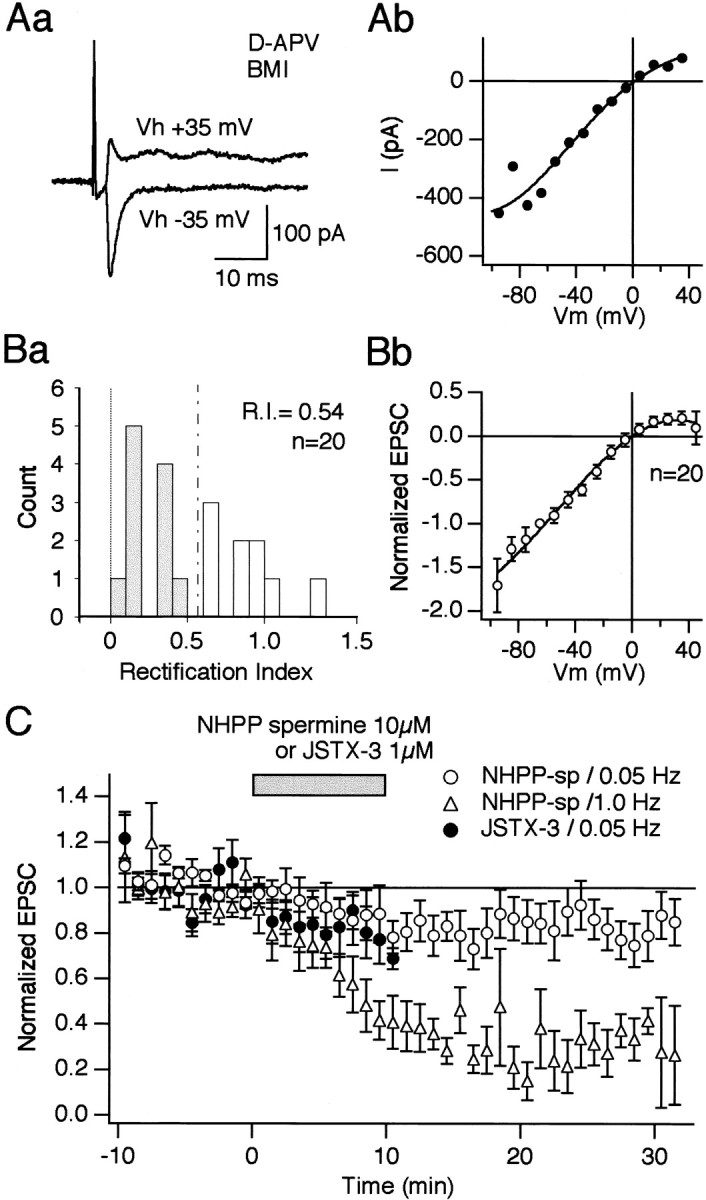
Striatal cholinergic interneurons possess Ca2+-permeable AMPA receptors. Aa, Evoked synaptic currents recorded from a cholinergic interneuron at holding potentials of +35 and −35 mV. Synaptic currents were recorded in the presence of d-APV (50 μm) and BMI (30 μm). Patch pipettes contained CsCl. Note that the current amplitude of the AMPA receptor-mediated EPSC at +35 mV was smaller than that taken at −35 mV. Ab, Current–voltage relationship of AMPA EPSC taken from the same cell shown in Aa.Ba, Rectification index (R.I.) is plotted for 20 cells. R.I. was defined as the conductance of the AMPA EPSC measured at +40 mV divided by the conductance at −70 mV. Thus, the R.I. of <0.57 (broken line) was regarded as inward rectifying. Eleven of 20 cells were considered cells containing Ca2+-permeable AMPA receptors (gray bars). The averaged R.I. was 0.54. Bb, The current–voltage relationship of normalized AMPA EPSCs obtained from 20 cholinergic interneurons shows inward rectification, indicating that this neuronal type as a whole possesses Ca2+-permeable AMPA receptors. The current amplitude taken at −60 mV was normalized to 1.0. The normalized values at each membrane potential were averaged across the cells and plotted against voltage to yield the averaged I–V curve.C, Use-dependent block of Ca2+-permeable AMPA receptors by NHPP-spermine and JSTX-3. Average normalized EPSC amplitudes monitored at 0.05 Hz are plotted from nine cells for NHPP-spermine (○) and seven cells for JSTX-3 (●). Application of both drugs caused ∼20% reduction of the normalized EPSC amplitudes when monitored at 0.05 Hz. By contrast, ∼70–80% reduction was observed in the case of NHPP-spermine at the same dose when test stimuli were applied at 1 Hz (▵,n = 6). After a stable baseline was obtained at a holding potential of −60 mV, NHPP-spermine and JSTX-3 were applied at 10 and 1 μm, respectively, during the time indicated by abar (10 min). Onset and offset times of drug application are shown in the graph (bar). It took ∼2 min for the solution to arrive at the recording chamber in our experimental configuration. Test stimuli were elicited at either 0.05 or 1 Hz. Differences of EPSC amplitudes between before and after NHPP-spermine at 0.05 Hz were statistically significant at 14, 16, 17, 20, 29, and 33 min after the start of bath application (paired t test,p < 0.05; n = 9). Differences of EPSC amplitudes between before and after NHPP-spermine at 1.0 Hz were statistically significant throughout 5 min after the start of bath application (p < 0.05 at 5 min,p < 0.01 thereafter, paired t test;n = 6). Differences between the experiment with NHPP-spermine at 0.05 Hz and that of 1.0 Hz were statistically significant (p < 0.0001, ANOVA).
