Fig. 6.
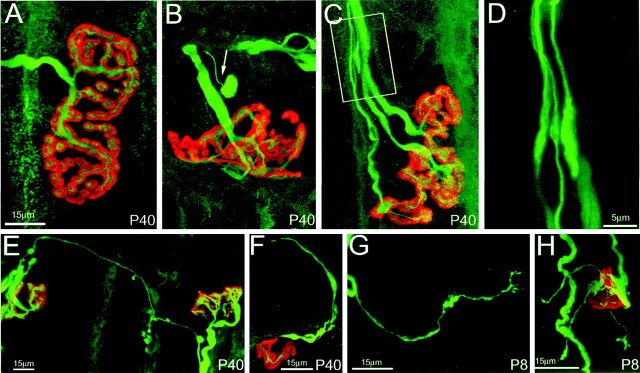
GDNF induces a dynamic state of axonal branching.A, Confocal reconstruction of a neuromuscular junction from control sternomastoid muscle at P40 shows that the junctions are large, singly innervated, and have a complicated pattern of receptor branches that accurately mirrors the branching pattern of the overlying terminal axon, which possesses no sprouts. B,C, In contrast, after 40 d of continual exposure to 2.0 μg/gm GDNF, junctions are often multiply innervated (C), smaller in area, and somewhat less complex than controls. Furthermore, many thin axons, terminating in bulbs, were evident (arrow, B). These structures are identical in appearance to “retraction bulbs” seen during naturally occurring synapse elimination in the first 2 postnatal weeks (Bernstein and Lichtman, 1999). Additionally, axons that ended in structures that resembled growth cones were observed in GDNF-exposed muscles (C, and enlargement shown in D).E, F, Also observed at P40 in GDNF-treated muscles were terminal sprouts that either connected nearby junctions (E) or exited neuromuscular junctions but ended blindly (F). G, H, Such sprouting was also seen at earlier ages. Shown here (G) is a blindly ending sprout off a YFP-labeled axon from a P8 spinotrapezius muscle in a mouse treated with GDNF since birth and a neuromuscular junction with three terminal sprouts (H) from the same animal, suggesting that throughout the duration of GDNF exposure axons were sending out new processes in the muscle. Scale bar shown in A is the same for B and C. In A–F, axons are labeled with anti-neurofilament antibodies (green), and postsynaptic acetylcholine receptors are labeled with rhodamine-α-bungarotoxin (red).