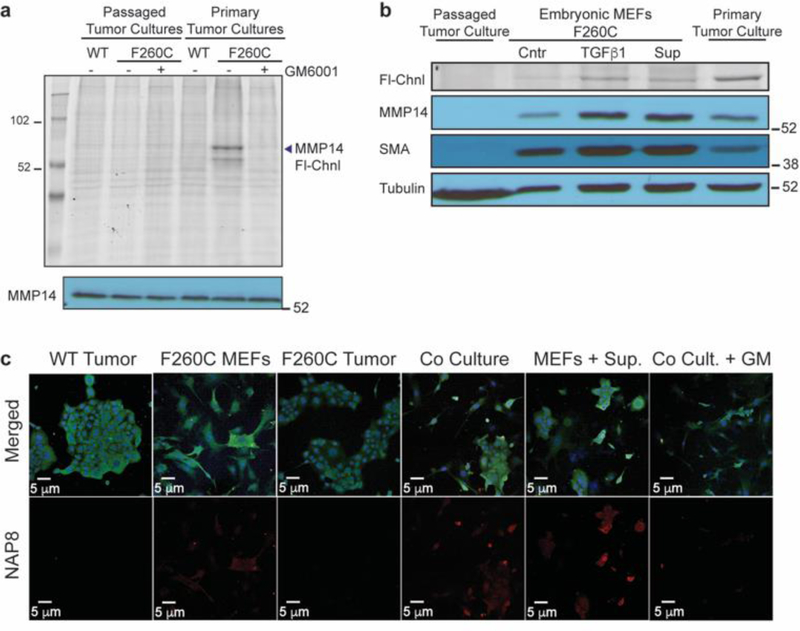
Figure 6:
The majority of MMP-14 activity originates from stromal cells in the microenvironment of late-stage carcinoma that respond to stimulation by tumor cells. a) Tumors harvested from the mammary glands of mMmp14(F260C)WTMMTV-PyMT+ and mMmp14(F260C)+/+MMTV-PyMT+ mice were cultured in 2D-cultures and treated with NAP8 (10 μM) at the first passage or after 10 passages. Whole cell lysates were analyzed by SDS-PAGE and scanned for in-gel fluorescence of Cy5 (Fl-Chnl). Western blot analysis was performed with MMP-14 antibody. b) Primary tumor cultures, passaged tumor cultures (neoplastic epithelial cells) and primary fibroblasts from mMmp14(F260C)+/+MMTV-PyMT+ mice were incubated with TGFβ1 (50 ng/ml) or supernatant of neoplastic epithelial cells (24 hours, serum free medium) for 24 hours, then treated with NAP8 (10 μM). Whole cell lysates were analyzed by SDS-PAGE and scanned for in-gel fluorescence of Cy5 (Fl-Chnl). Western blot analysis was performed with antibodies for MMP-14, α-smooth muscle actin (SMA) and α-tubulin. c) 2D-cultures of mMmp14(F260C)WTMMTV-PyMT+ and mMmp14(F260C)+/+MMTV-PyMT+ passaged tumor cells, fibroblasts and co-cultures of passaged tumor cells and fibroblasts were pretreated with DMSO or GM6001 (10 μM), or pre-incubated with supernatant of neoplastic epithelial cells (24 hours, serum free medium), then treated with NAP8 (10 μM). Cells were fixed and stained with anti-MMP-14 and DAPI and visualized by confocal microscopy. NAP8 (red), MMP14 (green), DAPI (blue), each experiment was performed in three independent biological repeats (scale bar 20 μm).