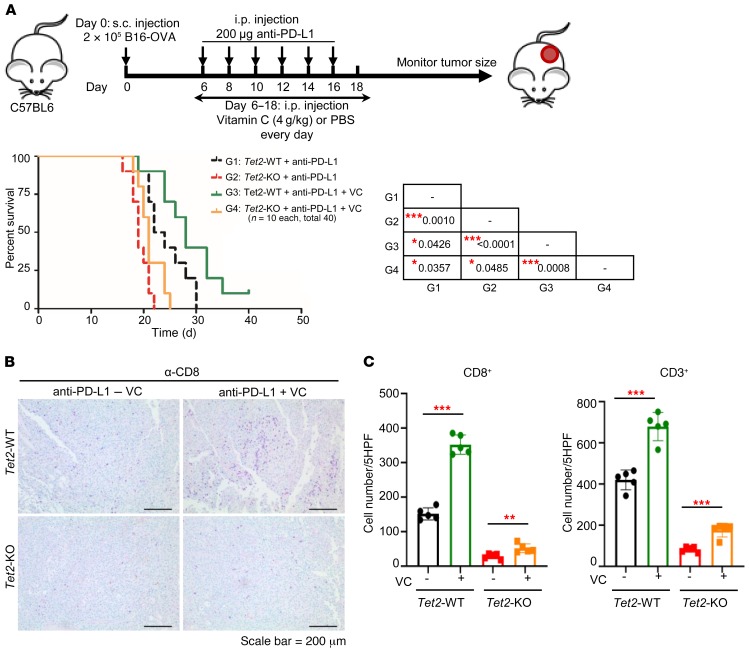
Figure 9. VC stimulates TET activity to enhance tumor-infiltrating lymphocytes and anti–PD-L1 immunotherapy.
(A) VC enhanced anti–PD-L1 immunotherapy. Kaplan-Meier survival curves for mice injected with WT or Tet2-KO B16-OVA cells and treated with anti–PD-L1 antibody and VC (sodium ascorbate) as indicated are shown. 2 × 105 WT or Tet2-KO B16-OVA cells, anti–PD-L1 antibody, and sodium ascorbate were injected s.c., i.p., and i.p., respectively, into C57BL/6 mice at the indicated time points. Kaplan-Meier survival curves for these mice are shown (n = 10 mice for each group). The survival curve of mice injected with WT or Tet2-KO B16-OVA cells with anti–PD-L1 treatment in Figure 1E is also shown by a dashed black or red line for reference. The P value was determined using log-rank (Mantel-Cox) test, comparing every 2 groups, and is shown in the table of the figure. *P < 0.05, ***P < 0.001. (B) VC enhanced tumor-infiltrating lymphocytes with anti–PD-L1 immunotherapy. CD8 immunostaining of Tet2-WT and -KO tumors from A treated with anti–PD-L1 antibody and VC is shown. Scale bar: 200 μm. (C) Quantification of CD8+ and CD3+ T cells from B and Supplemental Figure 7C. Average cell number per HPF is shown, with 5 HPFs calculated for each group. **P < 0.01, ***P < 0.001. Quantification of CD8+ and CD3+ T cells of mice injected with anti–PD-L1 in Figure 2, D and E, is also shown for reference. Error bars represent ± SD.