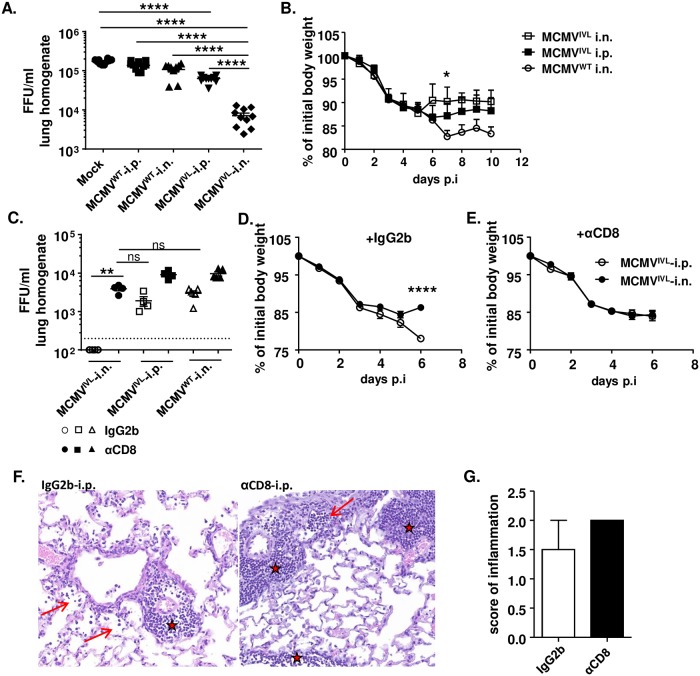
Fig 3. Intranasal immunization with MCMVIVL facilitates the elimination of IAV.
BALB/c mice were immunized with 2 x 105 PFU MCMVIVL or MCMVWT via the i.n. or i.p. route. Mock controls received 100 μl PBS by i.p. route. Once latency was established (> 3 months p.i), mice were challenged with IAV (PR8). (A) IAV titers in the lungs on day 5 post-challenge (i.n., 220 FFU) by focus-forming assay (FFA). Two independent experiments were performed and pooled data are shown, n = 10. (B) Body weight loss upon IAV challenge (i.n., 1100 FFU), n = 3–7. (C) CD8+ T cells were depleted systemically by i.p. injection of 200 μg anti-CD8α antibody (αCD8) or isotype control antibody (IgG2b) one day before PR8 challenge (i.n., 1100 FFU). Virus loads in the lung homogenates were quantified on day 6 post-challenge by FFA. Two independent experiments were performed and one cohort was shown. Each symbol represents one mouse, n = 5. Group means +/- SEM are shown. (D, E) Body weight loss upon IAV challenge (i.n., 1100 FFU) without (D) or with (E) systemic CD8+ T cell depletion. Two independent experiments were performed and pooled data are shown, n = 10. Group means +/-SEM are shown. (F) H&E staining of the lung tissue on day 5 post-challenge (i.n., 1100 FFU) with or without systemic depletion of CD8+ T cell. (G) The score of inflammation in the lungs upon IAV challenge (i.n., 1100 FFU). Bars indicate means, error bars are SEM. Significance was assessed by One-way ANOVA test or Two-way ANOVA test. *P<0.05, **P<0.01, ****P<0.0001, ns: no significant difference.