Abstract
Objective:
We hypothesized that intentional weight loss is associated with lower mortality risk, whereas unintentional weight loss is associated with higher mortality risk in chronic kidney disease (CKD).
Design and Methods:
We examined this hypothesis in 872 participants with age >20 years, body mass index ≥ 25 kg/m2 and CKD from 1999–2004 National Health and Nutrition Examination Survey who reported their 1 year prior and current weights and the intent to lose weight. We examined the association of self-reported intentional versus unintentional weight loss with all-cause mortality. Participants with no intent to lose weight and no change in weight were the reference group. A multivariable Cox regression model was used to relate mortality with intentional and unintentional weight losses after adjustment for demographics and comorbidity.
Results:
There were 446 deaths over 6271 years of follow-up. Compared to the reference group, intentional weight loss of 5% to <10% (hazard ratio (HR) 1.22, 95% confidence interval (CI): 0.74–1.99), intentional weight loss of ≥10% (HR 1.53, 95% CI: 0.75–3.12), and unintentional weight loss of 5% to <10% (HR 1.11, 95% CI: 0.71–1.75) were not associated with mortality; however, unintentional weight loss of ≥10% (HR 1.66, 95% CI: 1.06–2.58) was significantly associated with higher risk of mortality. Retrospective design and self-reported weight loss were the limitations.
Conclusions:
Intentional weight loss in CKD participants was not associated with lower mortality risk. This might reflect residual confounding. Mechanistic and interventional studies are warranted to determine the effects of intentional weight loss in CKD.
Introduction
OBESITY IS CONSIDERED one of the risk factors for the development and progression of chronic kidney disease (CKD).1–3 Both experimental and population studies have found mechanistic link between obesity and CKD independent of diabetes and hypertension.4
In general population, weight loss results in improvement in risk factors for cardiovascular disease, quality of life, glycemic control, and other obesity-related coexisting illnesses.5 However, the impact of weight loss on the risk of all-cause mortality and cardiovascular disease is not clear perhaps because of confounding from unintentional weight loss or methods used for weight loss.6–10 Given the mechanistic link between obesity and CKD, it is intuitive to consider the beneficial effect of intentional weight loss in CKD patient’s outcomes. In early CKD participants, weight loss achieved by various interventions including diet, exercise, lifestyle changes, pharmacologic treatment, and bariatric surgery have improved proteinuria and albuminuria, and normalized GFR, particularly if achieved by surgical interventions.11 However, there is very limited data evaluating the effect of weight loss on hard outcomes in CKD patients. A small nonrandomized observation study in patients with CKD and BMI >30 or >28 kg/m2 with comorbidities reported a longer event-free (death, myocardial infarction, stroke, and heart failure hospitalization) time over median follow-up of 32 months in the group who followed a 12-month weight loss program. The authors reported that participation in the weight loss program rather than weight loss alone was associated with longer event-free survival.12 Nonetheless, there is no large study evaluating the effect of weight loss on mortality in community dwelling population with CKD.
We hypothesized that intentional weight loss is associated with lower risk of mortality, whereas unintentional weight loss is associated with increased risk of mortality in CKD and examined this hypothesis in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) data set.
Materials and Methods
Study Population
The National Center for Health Statistics is conducting NHANES to obtain a sample of a representative population of the noninstitutionalized United States population. In the 1999–2004 NHANES data set, there were total of 8802 subjects who were ≥20 years and had BMI ≥25 kg/m2. Of these, weight loss and estimated glomerular filtration rate (eGFR) data were available in 8245 subjects. CKD was defined as estimated CKD-EPI eGFR <60 mL/minute/1.73 m2.14 The final cohort included 872 individuals who had CKD and in whom mortality statistics were available (Fig. 1).
Figure 1.
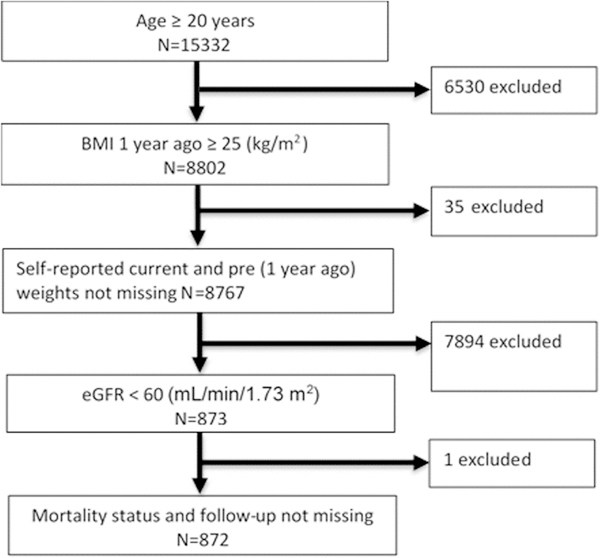
Flow chart of participant inclusion criteria. eGFR, estimated glomerular filtration rate.
Baseline Data
NHANES data collection details have been published elsewhere.13 Weight history obtained in this survey included the following questions: (1) How much do you weigh without clothes and shoes? (2) How much did you weigh a year ago? and (3) for those with >10 lb weight loss: Was the change between your current weight and weight a year ago intentional? Regardless of the weight change, all participants were asked whether they tried to lose weight in the past 12 months.
In the current analysis, the reference group comprised of participants who did not intend to lose weight and with no weight change (the difference between past year weight and current weight was between −5% and +5%). Weight gain group was defined as those who had ≥5% gain. Participants who reported an intention to lose weight were categorized further based on the degrees of weight loss as no weight change (between −5% and +5%), 5% to < 10% weight loss, and ≥10% weight loss. Unintentional weight loss groups (those who did not report an intention to lose weight but lost > 5% weight) were categorized into 5 to < 10% loss and ≥10% weight loss. In sensitivity analyses, similar weight change groups were defined using 10 to < 20 lbs and ≥20 lbs absolute weight loss.
Mortality Data
A Linked Mortality File through December 31, 2011 was created by the National Center for Health Statistics using a probabilistic match between NHANES and death certificate records from the National Death Index.15
Statistical Analysis
NHANES is based on a complex probability sample design. We used the svy suite of commands in Stata software, version 13 (Stata Corp., College Station, TX) and followed the analytical guidelines for NHANES data proposed by the Centers for Disease Control and Prevention. Hence, all results take survey weights into account.
Descriptive statistics were obtained for weight change groups including means and standard deviations (SDs) for numeric variables and proportions for categorical variables.
A multivariable Cox regression model was used to relate mortality with weight gain, intentional and unintentional weight loss groups. The reference group comprised of participants who did not intend to lose weight and with no weight change (the difference between past year weight and current weight was between −5% to +5%). The first model was adjusted for pre-BMI, baseline demographics (age, gender, race, education level), smoking, and alcohol use. The second model was further adjusted for comorbid conditions (myocardial infarction, congestive heart failure, stroke, lung disease, cancer, diabetes, and hypertension) and eGFR, which are potential confounders as well as intermediate variables in the causal pathway between weight loss and mortality.
These analyses were repeated with absolute weight (in lbs) change categories.
All analyses were performed with svy suite in Stata 14 (STATA Corporation, College Station, Texas).
Results
Baseline characteristics of 872 participants categorized by the 7 weight change categories are presented in Table 1. Supplemental table 1 presents similar data for the weight change in lbs. The reference category of no intent to lose weight and no weight change, by definition had stable BMI (29.6 ± 4.1 kg/m2 a year ago and 29.6 ± 4.2 kg/m2 at baseline). Weight gain group had a mean weight gain of 19.5 ± 9.4 lbs, and the BMI increased from 31.1 ± 3.9 kg/m2 to 34.4 ± 4.6 kg/m2. Those who intended to lose weight but did not lose weight had a modest −0.5 ± 3.6 lb change. However, those who intended to lose weight and lost weight between 5 to <10% and ≥10% had average weight losses of −15.0 ± 5.4 lbs and −33.6 ± 15.0 lbs, respectively. The unintentional weight loss groups also had comparable weight losses of −12.4 ± 3.8 lbs and −36.0 ± 16.1 lbs in the 5 to <10% and ≥10% weight loss groups, respectively.
Table 1.
Baseline Characteristics of CKD Participants by % Weight Change Groups (N = 872)
| Variable | Weight Gain | No Intent to Lose Weight, No Weight ⊿ |
Intent to Lose Weight |
Unintentional Weight Loss |
|||
|---|---|---|---|---|---|---|---|
| No Weight ⊿ | 5 to <10% Loss | ≥10% Loss | 5 to <10% Loss | ≥10% Loss | |||
| N = 117 |
N = 383 |
N = 176 |
N = 56 |
N = 35 |
N = 62 |
N = 43 |
|
| 14.7% | 39.8% | 23.1% | 7.7% | 4.5% | 5.5% | 4.7% | |
| Weight change (lb) | 19.5 ± 9.4 | 0.1 ± 3.0 | −0.5 ± 3.6 | −15.0 ± 5.4 | −33.6 ± 15.0 | −12.4 ± 3.8 | −36.0 ± 16.1 |
| Weight change (%) | 10.6 ± 5.1 | −0.02 ± 1.63 | −0.2 ± 1.9 | −7.2 ± 1.1 | −16.4 ± 6.4 | −7.0 ± 1.6 | −18.2 ± 6.8 |
| BMI 1 y ago (kg/m2) | 31.1 ± 3.9 | 29.6 ± 4.1 | 31.0 ± 4.1 | 34.4 ± 7.6 | 33.8 ± 4.5 | 30.1 ± 4.7 | 32.6 ± 4.9 |
| Baseline BMI (kg/m2) | 34.4 ± 4.6 | 29.6 ± 4.2 | 30.9 ± 4.1 | 31.9 ± 6.9 | 28.2 ± 4.1 | 28.0 ± 4.3 | 26.6 ± 4.1 |
| Age (y) | 66.9 ± 12.5 | 74.6 ± 9.6 | 68.8 ± 10.3 | 66.4 ± 11.0 | 68.2 ± 8.2 | 77.3 ± 9.1 | 72.3 ± 11.1 |
| Male (%) | 32.6 | 48.7 | 46.1 | 33.4 | 28.8 | 39.4 | 29.5 |
| African–American race (%) | 10.9 | 6.6 | 7.1 | 10.6 | 12.3 | 17.5 | 19.1 |
| Smoking (%) | 56.5 | 47.5 | 51.6 | 46.5 | 36.0 | 45.1 | 38.7 |
| Alcohol use (%) | 48.1 | 54.0 | 53.7 | 49.1 | 42.8 | 39.6 | 30.6 |
| ≥High school education (%) | 66.9 | 62.4 | 75.1 | 73.2 | 62.3 | 48.2 | 46.6 |
| Diabetes (%) | 23.8 | 27.0 | 25.9 | 33.0 | 44.4 | 20.2 | 24.2 |
| Hypertension (%) | 74.2 | 77.8 | 73.4 | 71.7 | 85.9 | 79.8 | 71.7 |
| Myocardial infarction (%) | 16.3 | 17.4 | 14.4 | 21.4 | 18.9 | 9.2 | 15.7 |
| Congestive heart failure (%) | 16.7 | 13.1 | 6.3 | 20.9 | 22.4 | 13.2 | 25.0 |
| Stroke (%) | 14.1 | 10.9 | 7.7 | 15.2 | 24.9 | 12.9 | 8.0 |
| Lung disease (%) | 16.6 | 10.3 | 12.4 | 22.4 | 13.2 | 12.9 | 18.0 |
| Cancer (%) | 20.6 | 25.8 | 21.8 | 25.1 | 18.2 | 19.3 | 6.3 |
| eGFR (mL/min/1.73 m2) | 46.4 ± 10.8 | 48.1 ± 10.6 | 48.3 ± 8.9 | 48.8 ± 9.1 | 46.9 ± 10.4 | 45.3 ± 12.3 | 42.4 ± 15.5 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Higher pre-BMI, age, and ≥ high school education were significantly associated with intent to lose weight in unadjusted logistic regression models, but only higher pre-BMI and age remained significant in a multivariate logistic regression model (Table 2).
Table 2.
Associations of Baseline Factors With Intention to Lose Weight
| Clinical Factors | Odds Ratio of Intent to Lose Weight (95% CI and P Value) |
|
|---|---|---|
| Unadjusted | Multivariate Model | |
| BMI 1 y ago (for each 1 kg/m2) | 1.08 (1.03 to 1.12, .001) | 1.06 (1.01 to 1.11, .01) |
| Age (for each 5 y) | 0.84(0.75 to 0.94, .003) | 0.87 (0.77 to 0.98, .03) |
| Male | 0.93(0.64 to 1.35, .71) | 0.87 (0.58 to 1.31, .51) |
| African–American race | 0.90 (0.63 to 1.28, .54) | 0.82 (0.53 to 1.26, .35) |
| Smoking | 0.99 (0.71 to 1.39, .97) | 0.96 (0.65 to 1.41, .82) |
| Alcohol use | 1.06(0.75 to 1.50, .72) | 1.03 (0.72 to 1.47, .89) |
| ≥High school education | 1.73 (1.11 to 2.70, .02) | 1.61 (0.97 to 2.66, .06) |
| Diabetes | 1.24 (0.87 to 1.77, .23) | 1.26 (0.86 to 1.85, .23) |
| Hypertension | 0.89 (0.59 to 1.36, .59) | 0.86 (0.56 to 1.33, .48) |
| Myocardial infarction | 1.01 (0.61 to 1.68, .97) | 1.25 (0.66 to 2.36, .48) |
| Congestive heart failure | 0.75 (0.50 to 1.13, .17) | 0.66 (0.41 to 1.07, .09) |
| Stroke | 1.00 (0.51 to 1.94, .99) | 1.20 (0.59 to 2.42, .61) |
| Lung disease | 1.21 (0.69 to 2.12, .51) | 1.19 (0.62 to 2.29, .60) |
| Cancer | 0.97 (0.63 to 1.48, .87) | 1.14 (0.73 to 1.77, .55) |
There were 446 deaths over 6271 years of follow-up. The number of deaths and the death rate in the weight change groups are provided in Figure 2. The mortality rate was the highest in the unintentional weight loss group irrespective of degree of weight loss.
Figure 2.
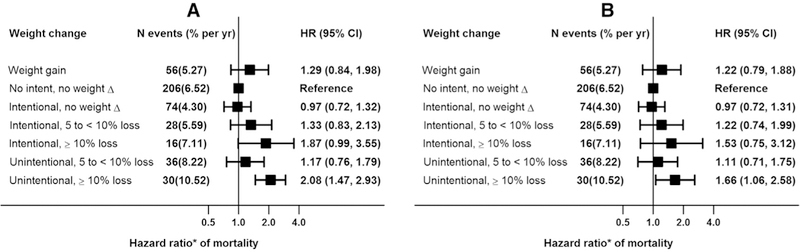
Mortality associations of intentional or unintentional weight loss (%) in CKD subpopulations. (A) Model 1 adjusted for pre-BMI, age, gender, race, smoking, alcohol and education; (B) Model 1 plus myocardial infarction, congestive heart failure, stroke, diabetes, hypertension, eGFR, lung disease and cancer. CKD, chronic kidney disease.
Compared to the reference group (no intent to lose weight and no change in weight), in a multivariate Cox regression model (Fig. 2, panel A) adjusted for pre-BMI, age, gender, race, smoking, and alcohol use, weight gain group had a nonsignificantly higher hazard of death (HR 1.29, 95% CI: 0.84–1.98). Compared to the reference group, HR for mortality in the intent to lose weight but no weight change group, intent to lose weight with 5 to <10% weight loss group, and intent to lose weight with ≥10% weight loss group were 0.97 (95% CI: 0.72–1.32), 1.33 (95% CI: 0.83–2.13), and 1.87 (95% CI: 0.99–3.55), respectively. With adjustment for factors that are potential confounders as well as intermediate variables, these associations attenuated further (Fig. 2, panel B).
In contrast, unintentional weight loss of ≥10% was significantly associated with increased mortality risk in both models (Fig. 2, panels A and B).
When analyses were repeated with weight loss defined as lbs, the pattern of results were similar (Fig. 3, panels A and B) for both intentional and unintentional weight losses.
Figure 3.
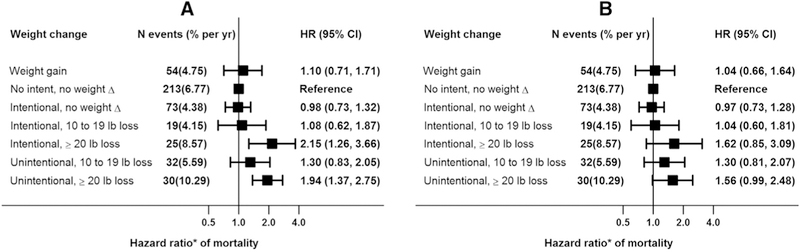
Mortality associations of intentional or unintentional weight loss (lb) in CKD subpopulations. (A) Model 1 adjusted for pre-BMI, age, gender, race, smoking, alcohol and education; (B) Model 1 plus myocardial infarction, congestive heart failure, stroke, diabetes, hypertension, eGFR, lung disease and cancer. CKD, chronic kidney disease; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Discussion
Among a nationally representative sample of US adults with CKD, unintentional weight loss ≥10% associated with higher risk of mortality. Contrary to our hypothesis, the intentional weight loss did not associate with lower risk of mortality in the CKD subpopulation.
In obese persons, alterations in the adipokines produced by adipose tissue result in metabolic derangements like insulin resistance, dyslipidemia, hypertension, and inflammation.16 Hence, higher body size in the general population has been associated with increased mortality.17 Although intentional weight loss in type 2 diabetes in the LOOK-AHEAD trial did not result in lower cardiovascular risk,8 a meta-analyses of randomized controlled trials of weight loss in the general population suggested a 15% decrease in mortality with intentional weight loss.18
However, the association of obesity with mortality in CKD population is not very straightforward. An analysis of Atherosclerosis Risk in Communities Study participants with CKD showed in those participants with BMI < 25 kg/m2, higher BMI was associated with lower risk of mortality.19 In contrast, no association was noted between BMI and mortality in participants with CKD and BMI of 25 kg/m2 or greater.19 In additional analysis, Madero et al20 also reported no association between sex-specific BMI quartiles and all-cause or cardiovascular mortality after a median follow-up of 10 years in 1,759 participants with mean age of 51 years and eGFR of 39 ± 21 mL/minute/1.73 m2 in the Modification of Diet in Renal Disease Study. In an analysis of US veterans,21 BMI <25 kg/m2 were associated with worse outcomes in all patients, independent of severity of CKD, but BMI 35 kg/m2 were associated with worse outcomes in patients with earlier stages of CKD, but this association was attenuated in those patients with eGFR<30 mL/minute/1.73 m2.
In a meta-analysis of observational and interventional studies of weight loss in CKD suggested that weight loss achieved by nonsurgical intervention is associated with significant improvement in proteinuria, and weight loss achieved by bariatric surgery in morbidly obese subjects is associated with normalization of glomerular hyperfiltration.11 In contrast, observational cohort studies reported increased risk of mortality with weight loss in CKD patients prior to initiation of dialysis22 and in patients on chronic maintenance dialysis.23–26 These observational studies of weight loss in CKD and dialysis patients are limited by the lack of data on whether the weight loss was intentional or unintentional,27,28 as unintentional weight loss might reflect an underlying comorbidity such as cancer or severe intercurrent illness.
Therefore, we conducted this analysis of NHANES data, which offers a unique opportunity to study mortality associations of intentional and unintentional weight loss in overweight or obese participants with CKD. Unintentional weight loss ≥10% or 20 lbs is associated with increased mortality risk.
On the other hand, intentional weight loss ≥10% or 20 lbs appears to associate with increased mortality risk with adjustment for pre-BMI, demographics, smoking, and alcohol use. However, with adjustment for comorbid conditions, these associations attenuated and reached nonsignificance. Nonetheless, contrary to our hypothesis, we did not note a lower risk of mortality with intentional weight loss in the CKD population. There are several possible explanations for these findings. First, these results could be because of confounding due to indication, that is, those who lose weight intentionally might have underlying (unmeasured) reasons that resulted in the intent to lose weight. While we tried to account for this with multivariable models, residual confounding cannot be excluded. Second, the methods used to achieve weight loss might influence mortality risk.8,29 A previous report suggested that a significant proportion of the CKD population use diets that may have high protein content and medications to promote weight loss that may be harmful.30 Third, weight loss might have resulted in significant loss of not only fat but also muscle which might increase the risk for death. A small nonrandomized observation study12 in patients with CKD and BMI >30 or >28 kg/m2 with comorbidities, patients assigned to 12-month structured weight loss program lost significantly more weight compared to control group and sustained a longer event-free (death, myocardial infarction, stroke and heart failure hospitalization) time over median follow-up of 32 months. However, on further analysis, it was the participation in the weight loss program which helped with the muscle mass, rather than weight loss alone which associated with longer event-free survival. Fourth, it could be speculated that catabolic pathways involved in weight loss might be harmful in those with CKD, but there is no evidence to support that notion at this time.
There were some important limitations to our study. First, the retrospective observational nature of the study precludes drawing any causal inferences. Multivariate models might not eliminate residual confounding. Second, weight loss and intent to lose weight were self-reported. It is conceivable that some with unintentional weight loss “claimed credit” by stating that weight loss was intentional. Third, most of the participants with CKD in NHANES reflect stage III CKD and not more advanced CKD.
Conclusion
Unintentional weight loss in overweight/obese participants is associated with increased mortality risk in CKD population. The observation that intentional weight loss in CKD participants was not associated with lower mortality risk warrants further mechanistic and interventional studies.
Supplementary Material
Acknowledgments
This work is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK091437 and R21 DK106574) and the University of Utah Study Design and Biostatistics Center (funded in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources).
Footnotes
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1053/j.jrn.2018.04.001.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the hypertension Detection and follow-up program. Am J Kidney Dis. 2005;46:587–594. [DOI] [PubMed] [Google Scholar]
- 2.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. [DOI] [PubMed] [Google Scholar]
- 4.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Guidelines on the Identification. Evaluation, and treatment of overweight and obesity in adults–the evidence report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed] [Google Scholar]
- 6.Williamson DF. Weight loss and mortality in persons with type-2 diabetes mellitus: a review of the epidemiological evidence. Exp Clin Endocrinol Diabetes. 1998;106 Suppl2:14–21. [DOI] [PubMed] [Google Scholar]
- 7.Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care. 2012;35:2613–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LOOK AHEAD Research GroupWing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009;22:93–108. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen MK, Hundrup YA, Obel EB, Gronbaek M, Heitmann BL. Intentional weight loss and mortality among initially healthy men and women. Nutr Rev. 2008;66:375–386. [DOI] [PubMed] [Google Scholar]
- 11.Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4:1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLaughlin HL, Hall WL, Condry J, Sanders TA, Macdougall IC. Participation in a structured weight loss program and all-cause mortality and cardiovascular morbidity in obese patients with chronic kidney disease. J Ren Nutr. 2015;25:472–479. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey. Available at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes03_04.aspx. Accessed June 22, 2017.
- 14.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from Serum Creatinine and Cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Center for Health Statistics. Linkage Methods and Analytical Support for NCHS Linked Mortality Data. Available at: http://wwwn.cdc.gov/nchs/nhanes/search/nhanes03_04.aspx. Accessed June 22, 2017.
- 16.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. [DOI] [PubMed] [Google Scholar]
- 18.Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One. 2015;10:e0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:992–998. [DOI] [PubMed] [Google Scholar]
- 20.Madero M, Sarnak MJ, Wang X, et al. Body mass index and mortality in CKD. Am J Kidney Dis. 2007;50:404–411. [DOI] [PubMed] [Google Scholar]
- 21.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stack S, Chertow GM, Johansen KL, Si Y, Tamura MK. Pre-ESRD changes in body weight and survival in nursing home residents starting dialysis. Clin J Am Soc Nephrol. 2013;8:1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabezas-Rodriguez I, Carrero JJ, Zoccali C, et al. Influence of body mass index on the association of weight changes with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–210. [DOI] [PubMed] [Google Scholar]
- 26.Molnar MZ. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant. 2011;11:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beddhu S If fat is good, muscle is better. Am J Kidney Dis. 2006;47:193; author reply 193–194. [DOI] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Kalantar-Zadeh K. Changes in body weight and subsequent mortality: are we any closer to knowing how to deal with obesity in ESRD? Clin J Am Soc Nephrol. 2013;8:1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 30.Navaneethan SD, Kirwan JP, Arrigain S, Schreiber MJ, Sehgal AR, Schold JD. Overweight, obesity and intentional weight loss in chronic kidney disease: NHANES 1999–2006. International J Obes (2005). 2012;36:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


