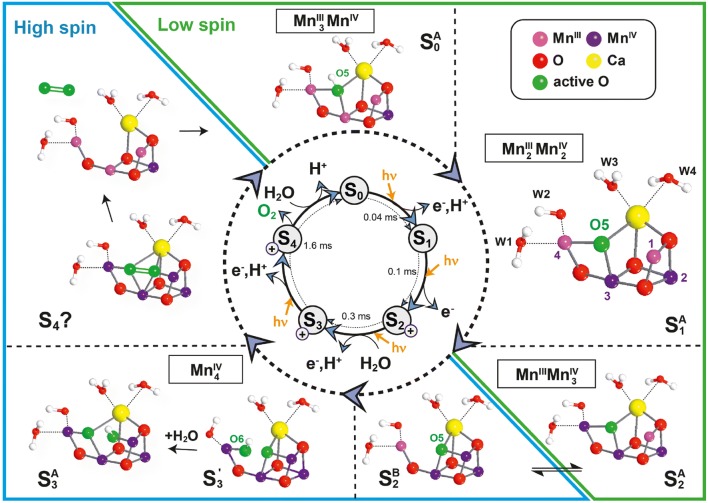
Fig. 8.
Model for the water oxidation cycle in PS II based on spectroscopic and theoretical work (Cox et al. 2015; Krewald et al. 2016) detailing the structures of the Mn cluster in the different S states, the water binding events and the O–O bond formation and O2 release in S4. The boxes show the determined oxidation states of the Mn ions in the respective S state. A color code is used for the assignment of the MnIII (light purple) and MnIV (dark purple) ions when the OEC is passing through the S states. Note that the oxygens of the waters and µ-oxo bridges are given in red except for the proposed substrate oxygens O5 and O6 (green). The S2 state exists in two conformations (closed/open cubanes), see text. S3 may also exist in an open (SA3) and a closed (SB3) cubane form (not shown). For S3 a state S3′ is shown in which manganese oxidation has occurred but the 2nd water has not been introduced. This state is present in native sample in a minority of PS II centers, but it is stabilized upon specific chemical treatment (methanol addition or Ca2+ to Sr2+ exchange) (Chrysina et al. 2019). A switching of the preferred total spin ground state configuration of the cluster is thought to take place in S2 from low to high spin and between S4 and S0 back from high to low spin; this is indicated by the diagonal line dividing the green and blue boxes. This switching of the spin state may be necessary for the formation of triplet dioxygen 3O2 in the final step of the cycle (see also Fig. 10).
Figure changed from (Krewald et al. 2016)