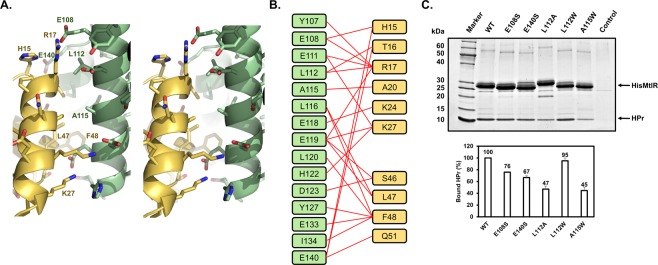
Figure 3.
The Binding interface between MtlR and HPr. (A) Stereoview of the MtlR-HPr interface. Proteins are color-coded as in Fig. 2. Amino acid side chains are shown in sticks. The amino acids subjected to the mutational study are labeled. (B) Schematic diagram denoting the amino acid interactions between MtlR and HPr. Red lines indicate the amino acid pairs in which the interatomic distance is less than 5 Å. (C) In-vitro binding assays of MtlR variants with HPr. Wild-type or mutant His-MtlR (200 μg each) was mixed with HPr (200 μg) in the binding buffer containing 20 mM Tris-HCl (pH 7.5), 50 mM NaCl, 2 mM β-mercaptoethanol and 5% glycerol and then subjected to Talon metal affinity chromatography (Clontech Laboratories, Inc.). After 3 washes with the binding buffer with 10 mM imidazole, the bound proteins were eluted with the binding buffer containing 150 mM imidazole. Eluted samples were analyzed by 4–20% SDS-PAGE followed by staining with Coomassie brilliant blue R-250. Representative data from three independent experiments are shown. The band intensities of bound HPr were analyzed using Multi Gauge version 3.0 software and normalized by the band intensities of each MtlR. The amount of HPr bound to each mutant MtlR was then expressed as a percentage of that bound to wild-type MtlR and shown below each lane.