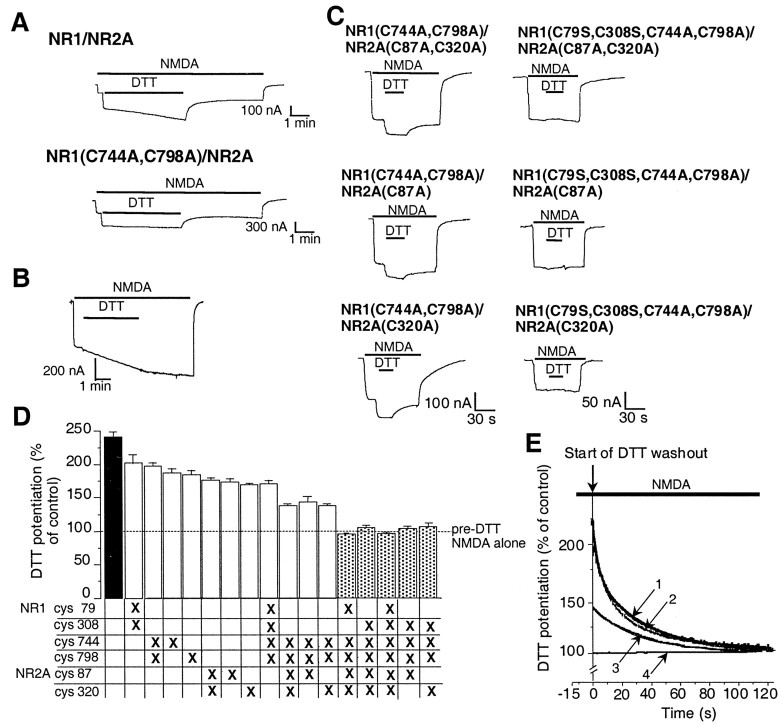
Fig. 2.
Identification of three pairs of cysteine residues involved in potentiation of NMDA-evoked currents by DTT.A, DTT potentiation of NMDA-evoked (200 μmNMDA plus 100 μm glycine) currents in an oocyte-expressing NR1–1a/NR2A receptors or NR1–1a(C744A, C798A)/NR2A receptors. DTT (3 mm) produced potentiation of rapid onset followed by a slow component of further potentiation in wild-type NR1–1a/NR2A receptors. Mutations of Cys 744 and Cys 798 in NR1 led to abolition of the slow component. However, the rapid potentiation by DTT was still present in NR1–1a(C744A, C798A)/NR2A receptors. Similar results were obtained from five oocytes for each group.B, DTT potentiation of NR1–1a/NR2A receptors in nominally Zn2+-free solution. In the presence of tricine (10 mm), added to the recording medium to produce a virtually Zn2+-free (0 Zn2+) solution, the slow component of DTT potentiation was still present, but the rapid effect of DTT was largely eliminated. C,Current tracings showing decreased DTT potentiation in oocytes expressing NR1–1a(C744A, C798A)/NR2A(C87A, C320A) receptors and complete absence of DTT potentiation in oocytes expressing NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors. Mutating either NR2A C87 or C320 alone had the same effect as mutating both cysteines together. D, Potentiation of NMDA-evoked currents by DTT in various cysteine mutant combinations expressed as a percentage of the response to NMDA alone (mean ± SEM,n = 4–9 for each group). The dashed line represents the absence of potentiation of NMDA-evoked currents. To ensure that predominantly the rapid component of DTT potentiation was considered, the NMDA-evoked currents were measured 15 sec after the onset of DTT and NMDA coapplication. DTT potentiation of all mutant combinations was significantly different from that of wild-type NR1–1a/NR2A, and potentiation of NR1–1a(C79S, C308S, C744A, C798A)/NR2A(C87A, C320A) receptors was significantly different from that of all other mutant combinations [p < 0.01, ANOVA followed by Fisher's protected least significant difference (PLSD) test]. E, Washout phase of DTT potentiation of NMDA-evoked currents in wild-type or mutant NR1–1a/NR2A receptors. Raw data are shown (traces 1–4) with exponential fits superimposed. After a 15 sec application of DTT, washout (beginning at t = 0) was observed for 120 sec. For display purposes, current traces are normalized to the NMDA response of each oocyte at 120 sec (arbitrarily defined as 100%). This plotting procedure does not display the slow, persistent component of redox modulation, which manifests a washout time constant of >10 min and which we have already shown is attributable to NR1 Cys 744 and Cys 798 (Sullivan et al., 1994). The first 120 sec of the washout phase of DTT potentiation in NR1–1a/NR2A receptors could be well fitted by the sum of two exponential kinetic components (trace 1 with time constants τ1and τ2; see Results for values). While mutation of NR1 Cys 744 and Cys 798 (trace 2) abolished the slow, persistent component (which is not manifest in this figure), it did not have any effect on the fast or the intermediate components (evidenced by the fact that traces 1 and2 are virtually superimposable). Additional mutations of NR2A Cys 87 and Cys 320 eliminated the fast component, but not the intermediate component (trace 3; which was well fitted by a single exponential curve with time constant, τ; see Results for value). If we also mutated NR1 Cys 79 and Cys 308, the intermediate component was also eliminated (trace 4; which can be fit by a straight line).