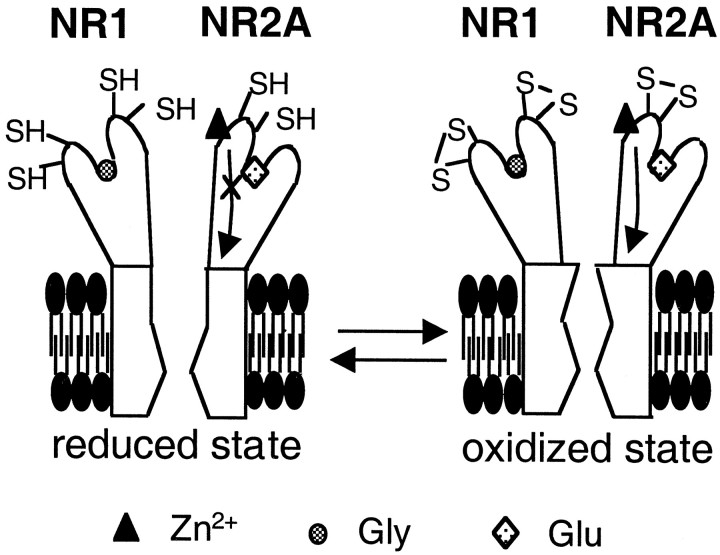
Fig. 7.
Proposed model for the redox-sensitive cysteine residues of the NMDA receptor underlying modulation of voltage-independent Zn2+ inhibition. It has been proposed that the glutamate-binding site is on the NR2A subunit (Laube et al., 1997; Anson et al., 1998), whereas the glycine-binding site is on the NR1 subunit (Kuryatov et al., 1994; Wafford et al., 1995; Hirai et al., 1996). In the oxidized state (right panel), three pairs of critical cysteine residues form disulfide bonds. In the oxidized state, the conformational change initiated by voltage-independent Zn2+ binding is transduced into greater inhibition of NMDA-evoked currents than in the reduced state. An arrow represents the transduction process mediating the inhibitory effect on channel activity after voltage-independent Zn2+ binding. In contrast, in the reduced state (left panel), disulfide bonds are broken to form free thiol groups. In mutated receptors lacking the critical cysteine residues, disulfide bonds also cannot be formed. Under these conditions, voltage-independent Zn2+binding is uncoupled from inhibition of NMDA-evoked currents. Uncoupling is diagrammed as a cross through thearrow representing the transduction process. Thus, there is less Zn2+ inhibition of NMDA current under reducing conditions. The observed results are counterintuitive if Zn2+ binding to cysteine residues were responsible for the inhibitory effect of Zn2+, because Zn2+ coordinates to free thiol and not to disulfide; Zn2+ would be expected to exert a greater inhibitory effect in the chemically reduced state of the receptor if Zn2+ binding to thiol were responsible for the effect, the opposite of the empirical result.