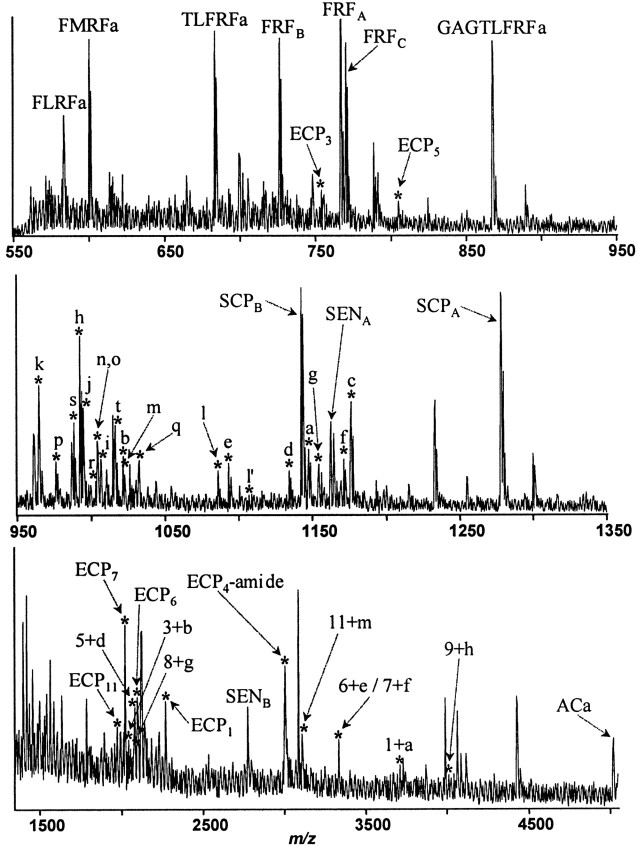
Fig. 3.
MALDI TOF mass spectrum (550–5000 Da range) from isolated buccal neurons of the rostral sensory cluster. Detection of the mass spectral peaks predicted by the putative amidated peptides on the enterin precursor confirm that they are in fact processed from the precursor. Assigned peaks are labeled with corresponding peptides. Peptides derived from five precursors, FMRFamide, FRFamide, SCP, sensorin, and enterin are also detected. Peaks corresponding to the products of the enterin precursor are shown with anasterisk. Lowercase letters signify the corresponding enterins, and numbers signify the corresponding enterin-connecting peptides (ECP). Peaks labeled with a letter plus numbercorrespond to amidated enterins still connected to their preceding ECP. Unamidated enterins were not detected. Enterins with a Gln at the N terminus (ENn, ENo, ENq, ENs, ENt) were detected only as a pyroglutamine form of the peptide. The enterin with a Glu at the N terminus (Enl) was detected as both E (labeled l′) and pE (labeled l) forms of the peptide. Peaks with masses corresponding to all of the 20 fully processed enterins were detected. Two of the enterins, ENn and ENo, are isomers and could not be distinguished on the basis of molecular mass. In addition, some ECP and ECP-enterin peptides were detected. One of the ECPs, ECP4, was detected as an amidated form (ECP4-amide).