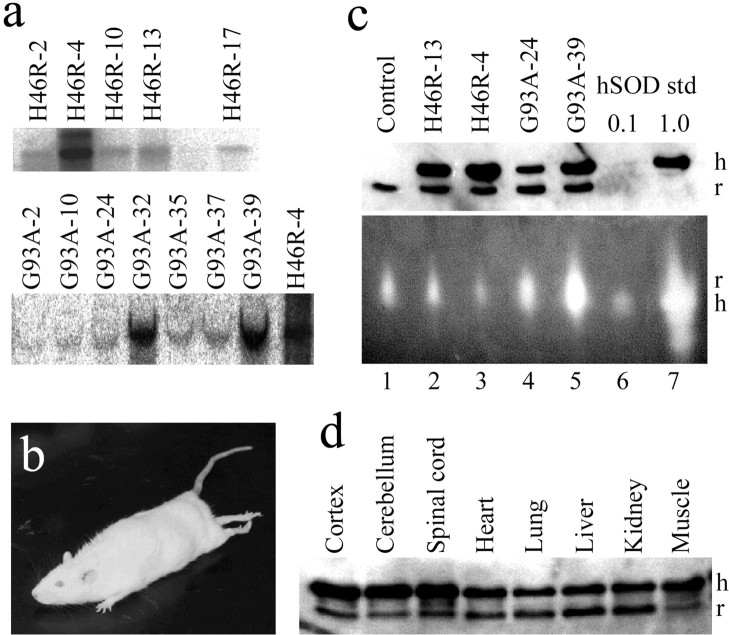
Fig. 1.
Analysis of human SOD1 transgene (H46R or G93A) copy number, protein expression, and SOD1 activity. a, Southern analysis of the human SOD1 gene in transgenic rats as determined by tail DNA blots. Five transgenic lines were established with the H46R mutation (top row), and seven transgenic lines were established with the G93A mutation (bottom row). The H46R-4 blot is shown in each row to allow comparison of the H46R and G93A results. b, An affected transgenic rat from the H46R-4 line demonstrates hindlimb weakness and abnormal posturing with segmental spasticity of the tail. c,Top panel, Quantitative immunoblotting of 4 μg of total protein extracts of spinal cord from nontransgenic littermate control, H46R lines (H46R-4, H46R-13), and G93A lines (G93A-24, G93A-39) using sheep polyclonal antibodies that recognize a common epitope shared between human (h) and rat (r) SOD1. Two 10-fold dilutions of purified human erythrocyte SOD1 (0.1, 1.0 U) were immunoblotted in parallel to provide standards for quantitation. Bottom panel, SOD1 enzymatic activity in spinal cord extracts (20 μg of protein) from the same nontransgenic littermate control or transgenic rats determined on native gels. Note that the electrophoretic migration of rat SOD1 (r) differs from that of human erythrocyte SOD1 (h). d, Total protein (4 μg) from various tissues from 2-month-old transgenic H46R-4 rats was immunoblotted with the same sheep polyclonal antibody as inc, recognizing human and rat SOD1.