Abstract
Objectives
Fel d1 is a major allergen that may affect humans sensitive to cat allergens, and it can be detected in the saliva and on the hair of cats. We studied the variability of salivary Fel d1 in typical house cats (ie, neutered domestic shorthair cats) and the factors that could be associated with that variability.
Methods
Saliva samples were collected from 64 cats, twice daily, every other day, for a year, at two locations (Missouri, USA, and Ontario, Canada). Salivary Fel d1 levels were measured using an immunoassay. Correlations and linear mixed-effects model analyses were run to assess which factors significantly affected the Fel d1 levels.
Results
Salivary Fel d1 levels varied significantly both within and among cats. Cat averages over the year ranged from 0.4–35 µg/ml, and a higher average correlated with a higher SD (P <0.001). The first collection of the day tended to be higher than the afternoon collection (P <0.001). Sex, coat color or body size did not relate to cats’ average Fel d1 production, but older cats tended to have lower salivary Fel d1 levels (P <0.001). Fel d1 levels from four samples were reliable in identifying cats producing stable low levels of Fel d1.
Conclusions and relevance
We observed a wide and continuous range of salivary Fel d1 production in domestic shorthair cats. In particular, a subset of cats had stable low levels throughout the course of the year, and they can be identified by analyzing a few saliva samples rather than their physical appearance.
Keywords: Allergens, immunoassay, Fel d1, saliva
Introduction
Worldwide, 10–15% of adults are estimated to be sensitized to cat allergens, making this one of the most common allergen sensitivities. Allergens trigger the production of IgE in sensitive individuals. 1 Cats produce several allergens known to react with human IgE: the secretoglobin Fel d1, Fel d2/albumin, Fel d3, the lipocalin Fel d4 and Fel d5. 2 Fel d1 is the most extensively characterized and it is considered to be the single most potent cat allergen. 3 Indeed, >90% of individuals sensitized to cat allergens have IgE directed against Fel d1, and for many of them Fel d1 was the only feline allergen to which they were sensitized. 4
The main reservoirs of Fel d1 in cats are saliva and hair, and the allergen is produced predominantly in sublingual and sebaceous glands, although anal and lachrymal glands also produce it.5–7 Fel d1 was first identified four decades ago and its protein structure described two decades ago; however, its biological function is still not clear.8–13 Many studies have investigated the presence of Fel d1 in the environment (schools, public transport, offices, houses), as well as efforts to mitigate its effect on humans.14–25 Additionally, a few studies addressed a potential link between Fel d1 levels and physical attributes of cats, in particular sex and reproductive status.26–28
Several feline breeds have the reputation of being hypoallergenic, such as the Siberian or Balinese, and an often cited ownership survey reported light-colored cats as being less likely to cause allergen-related sensitivities.29,30 However, the scientific data supporting particular cats as hypoallergenic is lacking. 31 Hence, the recommendations for cat-allergen sensitized patients include various medications and avoidance of allergens. 32 Human sensitivities to cat allergens are cited as an important factor in cats being relinquished or returned to shelters. 33
We hypothesized that a better understanding of Fel d1 levels in cats could be key in proposing options to households where cat allergy is a concern. We used a non-invasive saliva collection method in order to measure salivary Fel d1, and our preliminary results showed great variability among cats (unpublished data). Neutered domestic shorthair cats are the most common according to pet ownership surveys.34,35 Therefore, we carried out a large-scale study with neutered domestic shorthair cats, and we measured salivary Fel d1 throughout a year. Our goal was to characterize the expected range of salivary Fel d1 and to investigate factors that could be associated with Fel d1 levels, such as a cat’s physical attributes.
Materials and methods
The trial protocol was reviewed and approved by the Nestlé Purina Pet Care and Use Committee and in accordance with national and international guidelines of animal care.
Study cats
Healthy domestic shorthair cats, only neutered adults, were enrolled within two different research colonies. Group 1 included 27 cats (Missouri, USA) and group 2 included 37 cats (Ontario, Canada). An identical trial protocol was used and sample collections started in the same year (Table 1). All cats were housed indoors in a temperature and humidity controlled environment. They had ad libitum access to their food portion and to water. They were weighed and assigned a body condition score on a 9 point scale, except for eight cats in group 2. 36 At enrollment, cats ranged in age between 1.2 and 15.3 years of age, and ranged in body weight between 3 kg and 9 kg (Table 1).
Table 1.
General information on study populations at both locations (average and range)
| Group 1 (Missouri) | Group 2 (Ontario) | |
|---|---|---|
| Number of cats | 27 | 37 |
| Sex (all cats neutered) | 7 females; 20 males | 30 females; 7 males |
| Age (years) | 7.8 (1.2–15.3) | 8.7 (2.5–14.4) |
| Weight (kg) | 5.9 (3.0–9.0) | 5.2 (3.4–8.1)* |
| Body condition score (scale 1–9) | 6.3 (5–8) | 7.3 (5–9)* |
| Number of saliva collections per cat | 250 (53–324) | 297 (241–313) |
| Collections started in | Spring | Fall |
Data are mean (range) where relevant
No data for eight cats
Coat attributes
Coat attributes were categorized as main coat color, other coat color(s) and coat pattern. To compare with previous studies,29,37 we also categorized the main coat color as dark (black, blue or brown) and light (cream, orange, silver or white); calico cats (calico, torbie or tortoiseshell) were excluded from this classification owing to their mixture of hair coloring. Coat pattern was classified as either tabby or other.
Sample collection and Fel d1 analysis
Saliva was collected twice daily, every other day, for 1 year. The first saliva collection was performed just before feeding (mid-morning), and the second saliva collection 3 h later (early afternoon). Not all saliva collections were performed as planned; the causes of interruptions included unrelated health issues, moving cats to a new building and removing cats owing to their enrollment in concurrent studies.
Saliva collection was performed with a commercially available Salivette (Sarstedt) following the manufacturer’s instructions. The cats were allowed to chew on the Salivette for about 10–15 s, then the Salivettes were centrifuged (1000 g for 2 mins) to obtain the saliva.38–40 Samples were stored at −80°C and shipped on dry ice until analysis. Samples were analyzed using a commercial Fel d1 ELISA kit (Indoor Technologies) and the results were expressed in µg Fel d1 per ml saliva.
Statistical analysis
The Fel d1 data followed a log-normal distribution. We conducted statistical analyses on both transformed and non-transformed data. The results were similar either way, indicating that our analyses were robust to the non-normally distributed data. To help with interpretability, we reported results on non-transformed data. Our statistical analyses gave similar results whether the two groups were analyzed separately or combined. Considering the different distribution of Fel d1 between group 1 and group 2, results from the two groups were analyzed and reported separately.
Pearson parametric correlation was performed between the average and the SD of salivary Fel d1 for each cat.
Factors that could influence Fel d1 levels were investigated with linear mixed-effects model analysis. 41 Because the Fel d1 levels were influenced by which cat they originated from, cat identification was entered as a random effect in all our models, meaning the intercept was allowed to vary among cats. Several models had two categories (eg, female and male). Two of the coat color models had multiple categories, although we did not include categories with small sample size (defined as <5% of the total number of cats in the model). Weight and age were run as continuous variables in their respective models. When examining the impact of age on Fel d1 levels, number of days in the study was also included as a random effect. These results were reported as beta (β) – the estimated population average differences based on the model, and a P value was provided for the overall model. P values were obtained by using Satterthwaite approximations of degrees of freedom.
Discriminant function analysis was performed to investigate how many samples would be required to successfully identify cats with low stable levels of Fel d1, whether based on average, or average combined with SD. We defined these low-producer cats as the lower third of the production range, which corresponded in our dataset to a yearly average of <4 µg/ml.
All statistical analyses and graphing were conducted in R, using R core (R Core Team, 2012) and using lme4, or in GraphPad Prism 5.02 for Windows (GraphPad Software). Values were reported as average ± SD, and the results of linear mixed-effects models as estimate ± SE. Statistical significance was set at P value <0.05.
Results
Variability of salivary Fel d1
On average, 250 saliva samples per cat were analyzed for group 1 and 297 saliva samples per cat for group 2 (Table 1). The average Fel d1 salivary level was 6.3 ± 7.8 µg/ml in group 1 (27 cats) and 8.1 ± 12.8 µg/ml in group 2 (37 cats). Salivary levels ranged from 0.05–103.1 µg/ml in group 1 (6760 values), and from undetected to 322.1 µg/ml in group 2 (10,981 values).
Intra-cat variability of salivary Fel d1
Large differences in salivary Fel d1 levels were noted throughout the course of the year for each cat: the average coefficient of variation (CV) within cats was 57% for group 1 and 85% for group 2 (Tables S1 and S2 for individual CVs; see the supplementary material). Additionally, the intra-cat SD was correlated to their average Fel d1 level (r = 0.98 for group 1 and r = 0.93 for group 2 [P <0.001]; Figure 1). Therefore, cats with low average Fel d1 tended to have low variability, meaning their levels remained consistently low over a year. In contrast, cats with high average Fel d1 levels tended to have high variability.
Figure 1.
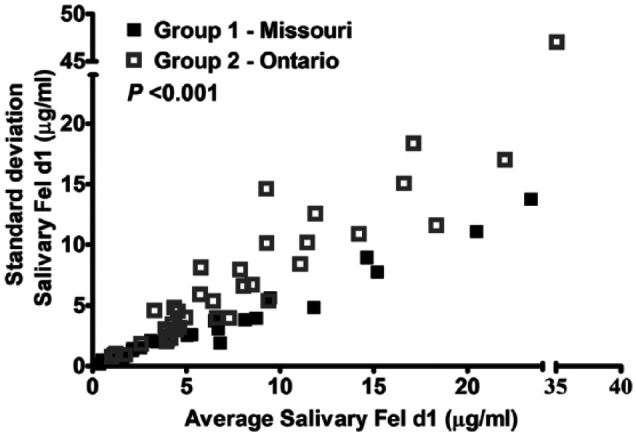
Correlation between average and SD of salivary Fel d1 for each cat (n = 64; r = 0.98 for group 1 and r = 0.93 for group 2 [P <0.001])
Inter-cat variability of salivary Fel d1
In addition to intra-cat variability, the variability in salivary Fel d1 was also high among cats, and that is best summarized by a cat’s yearly salivary Fel d1 average. The mean of the cats’ yearly averages was 6.2 ± 6.1 µg/ml in group 1 (27 cats) and 8.2 ± 6.7 µg/ml in group 2 (37 cats). The lowest yearly average for a cat was 0.4 µg/ml and the highest 35.0 µg/ml; the difference between these two cats’ yearly averages was greater than 80-fold (Tables S1 and S2 in the supplementary material).
Distribution of salivary Fel d1
Salivary Fel d1 exhibited a positively skewed distribution both at the individual level (Table S1 and Table S2 in the supplementary material) and at the population level (2.94 for group 1 and 9.5 for group 2). In other words, many Fel d1 values were relatively low, whereas a small number of values were extremely high.
Linear mixed-effects model analyses
Salivary Fel d1 levels in relation to time and/or experimental factors
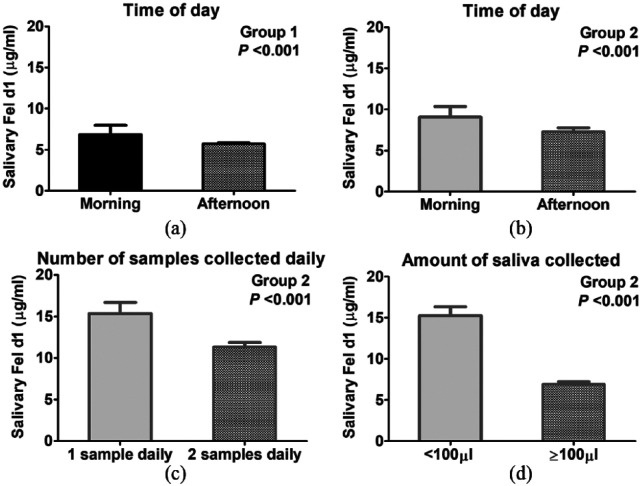
The first saliva collection was performed in the morning, and there was a significant trend in both group 1 (β = −1.08, P <0.001) and group 2 (β = −1.76, P <0.001) for higher Fel d1 levels in the morning than in the afternoon (Figure 2a,b).
Figure 2.
Graphical representation of linear mixed-effects models for salivary Fel d1 in relation to time of day of sample collection in (a) group 1 (n = 27; P <0.001) and (b) group 2 (n = 37; P <0.001), in relation to (c) sampling once or twice daily in group 2 (n = 37; P <0.001) and in relation to (d) amount of saliva collected in group 2 (n = 37; P <0.001); estimates ± SE
Concurrently, we investigated if the higher variability observed in group 2 (Ontario, Canada) could be explained by experimental factors. This was the first saliva collection study at this location, and we noticed that, occasionally, one out of the two daily collections was absent. Additionally, several samples had very low saliva volumes, particularly at the beginning of the study. In group 2, collecting once (β = −4.02, P <0.001) or collecting <100 µl saliva (β = −8.33, P <0.001) both resulted in higher Fel d1 levels (Figure 2c,d). For example, the 322 µg/ml value was obtained for cat 64 during the first week of collection, and that sample had <100 µl saliva collected. In other words, time effects (eg, time of day, time of year) may be confounded with experimental factors (eg, number of collections in a day, variations in amount of saliva collected).
The Fel d1 levels varied throughout the year, as illustrated by the monthly salivary Fel d1 production of two cats from group 1 (Figure 3). We could not run seasonality models without experimental confounding factors complicating the interpretation of the results (data not shown).
Figure 3.
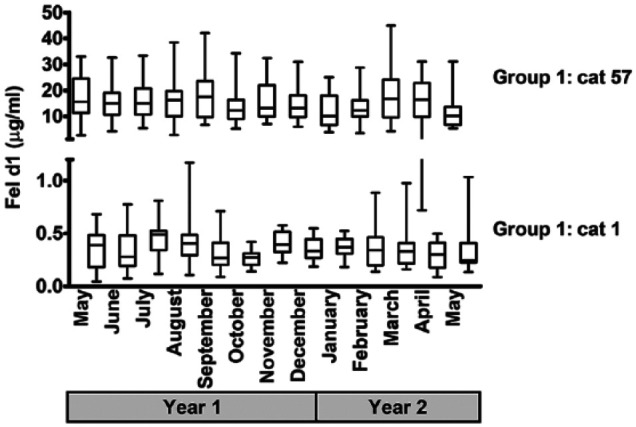
Yearly variability of salivary Fel d1 (µg/ml) for two cats in group 1 (Missouri); box plots represent minimum, median and maximum; y-axis presented in two sections in order to see box plots of cat 1
Salivary Fel d1 levels in relation to cats’ physical attributes
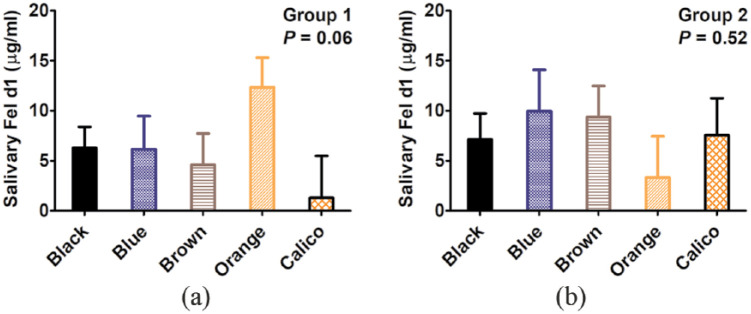
As shown in Table 2, there were no significant differences between castrated males and spayed females for either group 1 (P = 0.08) or group 2 (P = 0.29). Similarly, the cat’s weight and the salivary Fel d1 levels were not related (P = 0.44 for group 1, P = 0.53 for group 2 [Table 2]). There were no significant differences between the various main coat colors (P = 0.06 for group 1, P = 0.52 for group 2 [Figure 4]), between dark- and light-colored cats (P = 0.33 for group 1, P = 0.38 for group 2, Table 2) or tabby and non-tabby coat pattern (P = 0.57 for group 1, P = 0.48 for group 2 [Table 2]).
Table 2.
Results of linear mixed-effects models for salivary Fel d1 in relation to cats’ physical attributes
| Estimate beta (β) | SE | Degrees of freedom | T value | P value | |
|---|---|---|---|---|---|
| Sex | |||||
| Group 1 – Missouri | |||||
| Intercept | 2.87 | 2.14 | 27.02 | 1.34 | |
| Sex (male) | 4.59 | 2.49 | 27.03 | 1.85 | 0.08 |
| Group 2 – Ontario | |||||
| Intercept | 7.62 | 1.20 | 37.01 | 6.37 | |
| Sex (male) | 2.98 | 2.74 | 36.98 | 1.09 | 0.29 |
| Weight | |||||
| Group 1 – Missouri | |||||
| Intercept | 11.04 | 6.03 | 23.99 | 1.83 | |
| Weight (kg) | −0.80 | 1.02 | 24.00 | −0.78 | 0.44 |
| Group 2 – Ontario | |||||
| Intercept | 12.65 | 5.57 | 29.01 | 2.27 | |
| Weight (kg) | −0.69 | 1.09 | 29.00 | −0.63 | 0.53 |
| Age | |||||
| Group 1 – Missouri | |||||
| Intercept | 20.81 | 2.43 | 39.05 | 8.45 | |
| Age (years) | −1.74 | 0.20 | 159.31 | −8.67 | <0.001 |
| Group 2 – Ontario | |||||
| Intercept | 68.97 | 6.50 | 60.35 | 10.62 | |
| Age (years) | −6.56 | 0.64 | 106.63 | −10.29 | <0.001 |
| Coat base color – dark vs light | |||||
| Group 1 – Missouri | |||||
| Intercept | 5.70 | 1.54 | 25.05 | 3.71 | |
| Color (light) | 2.42 | 2.43 | 25.02 | 0.99 | 0.33 |
| Group 2 – Ontario | |||||
| Intercept | 8.90 | 1.41 | 31.01 | 6.30 | |
| Color (light) | –2.66 | 2.97 | 30.99 | −0.90 | 0.38 |
| Coat pattern | |||||
| Group 1 – Missouri | |||||
| Intercept | 5.17 | 3.53 | 22.96 | 1.46 | |
| Pattern (tabby) | 2.17 | 3.79 | 23.00 | 0.57 | 0.57 |
| Group 2 – Ontario | |||||
| Intercept | 7.16 | 1.66 | 34.01 | 4.32 | |
| Pattern (tabby) | 1.62 | 2.28 | 34.00 | 0.71 | 0.48 |
Beta = slope of the effect; SE = SE of the slope of the effect; degrees of freedom = degrees of freedom of the slope of the effect; T value = T value of the slope of the effect; P value = P value of the slope of the effect
Figure 4.
Graphical representation of linear mixed-effects models for salivary Fel d1 in relation to main coat color: (a) group 1 (n = 27; P = 0.06) and (b) group 2 (n = 37; P = 0.52); estimates ± SE. Sample size for cream, silver and white colors too small to include in the model
Only age was found to be significant (Figure 5; Table 2) as older cats often had lower levels of salivary Fel d1 in both group 1 (β = −1.74, P <0.001) and group 2 (β = −6.56, P <0.001).
Figure 5.
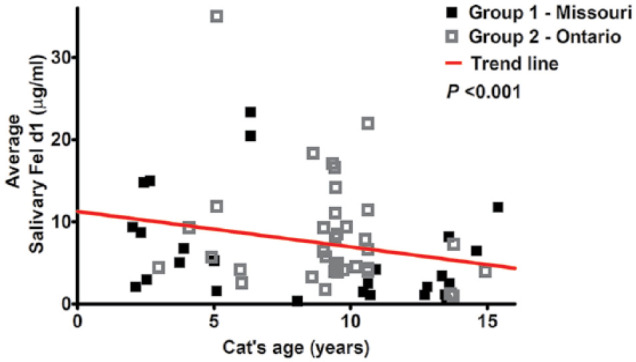
Graphical representation of linear mixed-effects model for salivary Fel d1 in relation to a cat’s age; trend line and P value obtained for groups 1 and 2 combined (n = 64; P <0.001)
Identification of low-producer cats
Some cats produced less salivary Fel d1 throughout the year (Figure 1). Considering many cats presented occasional low Fel d1 values (Tables S1 and S2 in the supplementary material), we investigated how many saliva samples were required to identify accurately these low-producer cats. Using the average and SD, four saliva samples had a 91% success rate in correctly identifying them (Table S3 in the supplementary material). Furthermore, the classification gained in accuracy if the values were randomly selected in our data set; values that were selected sequentially tended to be less accurate owing to the yearly variability (Figure 3).
Discussion
Variable levels of the Fel d1 allergen have been reported in the environment, such as in schools, public transport, offices and houses.14–25 Our study centered on the variability of Fel d1 in cats’ saliva: multiple measurements were obtained throughout a year in a non-invasive manner from a large number of cats. To our knowledge, this is the first study with so many direct Fel d1 measurements from cats, and our findings emphasize a biological variability to take into account in future studies.
We observed a wide and continuous range of salivary Fel d1 levels, and they were first and foremost influenced by which cat they originated from. Yearly averages ranged from 0.4–35 µg/ml, and levels for a given cat were variable, whether day-to-day or throughout a year, with coefficients of variation of about 40–150% (Tables S1 and S2 in the supplementary material). Furthermore, a high yearly average correlated with a higher variability (P <0.001; Figure 1). Interestingly, our results are in line with an early study with 12 cats in which an air sampler was used to measure Fel d1 being shed into the room: the researchers observed a high variability within and among cats, and the low-producer cats presented the least variability. 26 The positive skewness of our data indicated that all cats could present low levels of Fel d1 at some sampling points, even cats with a high yearly average (Tables S1 and S2 in the supplementary material). We studied domestic shorthair cats, as being typical pet cats and not specifically breeds cited as being hypoallergenic.30,33,35 Yet a subset of them had stable low levels of Fel d1 throughout the course of the year, as illustrated by cat 1 in Figure 3.
Could a cat’s physical attributes – such as sex, coat color or body size – be related to their salivary Fel d1 levels? Sex (P = 0.08) or coat color (P = 0.06) seemed close to significance in group 1 (27 cats), but these trends were not found in group 2 (37 cats). We concluded there was no impact of sex among neutered cats. Previous studies reported higher Fel d1 levels in male cats,26,42 decreased levels in males after castration, 43 higher levels only in neutered cats, 28 higher levels only in intact males vs females or neutered cats, 27 or no difference among neutered cats. 37 Differences in the number of cats enrolled, the collection method or the inclusion of intact animals could explain these various and sometimes contradictory results, as well as the inherent variability in Fel d1 among and within cats. Coat color has been suggested previously, although not confirmed subsequently, and we showed that neither coat color nor coat pattern could predict Fel d1 levels (Figure 5; Table 2).29,37 With regard to body size, the cats ranged from 3–9 kg, and we found no relation between a cat’s weight and salivary Fel d1 levels (Table 1 and Table 2). A study measuring Fel d1 in house dust also showed no correlation to a cat’s body size. 28 Ultimately, age was the only attribute in our study that related to salivary Fel d1 levels: older cats tended to have lower Fel d1 levels (Figure 5). One other study considered the cat’s age and found no differences, so this relationship still needs to be clarified. 44
It was not clearly established if Fel d1 levels in saliva could be related to Fel d1 levels measured on hair, or to environmental levels such as in the room a cat is located. Saliva is one of the reservoirs from which the Fel d1 allergen gets deposited on hair and subsequently shed into the environment, 1 so we hypothesized these levels may be related. Furthermore, we have a data subset showing that Fel d1 levels on hair significantly correlated to average salivary levels, even with a year’s gap between the two types of collection (unpublished data). This would be a key point to investigate because allergen dose thresholds – based on Fel d1 levels in dust reservoirs in homes – have been proposed for sensitization and for symptom exacerbation in humans. 45
Under the hypothesis that salivary levels could be related to environmental levels, we investigated how to identify cats producing stable low levels of salivary Fel d1. Most of the physical attributes we investigated could not be linked to Fel d1 levels. Moreover, owing to the inherent variability of Fel d1 within and among cats over time (Figures 1 and 3), a single saliva sample should not be used to estimate a cat’s average Fel d1. However, the average and SD of four saliva samples, ideally not taken on consecutive days, would have about 90% success in correctly identifying a low-producer cat (Table S3 in the supplementary material).
We showed the morning collection was more likely to be higher (P <0.001) than the afternoon one, but that could be due to it being the first collection of the day rather than a time-of-day effect (Figure 2a,b). Indeed, experimental factors, such as collection frequency or saliva amount collected, were confounding the results in this study design (Figure 2c,d). So although the study spanned a year and generated a large data set of Fel d1 values, and although there were significant differences throughout the year (data not shown), we could not interpret the seasonal differences of the Fel d1 allergen in saliva.
Conclusions
We described the variability of the Fel d1 allergen in cats’ saliva, which gives valuable insight into why relying on single sampling or very few cats may give unreliable results. Furthermore, we proposed a way to identify cats producing low stable levels of salivary Fel d1, but it is not known how salivary and environmental levels of Fel d1 relate to each other, so further research is required. Nevertheless, a common recommendation for sensitized individuals living with a cat is to reduce environmental levels of Fel d1, for example by vacuuming regularly, so a low-producer cat could be a great asset in this scenario.
Supplemental Material
Summary of salivary Fel d1 values for group 1 (Table S1). Summary of salivary Fel d1 values for group 2 (Table S2). Success (%) of correctly identifying low-producer cats based on their average salivary Fel d1 level only, or combined with their SD, in relation to the number of samples collected (Table S3)
Acknowledgments
We would like to thank: Peichuan Sun for developing the saliva collection method; the teams at both locations for collecting and shipping the samples; the Nestlé Purina Immune Lab – in particular Christina Toenjes – for inventorying the samples and running the immunoassays; Dr Qinghong Li for his input on the data analysis; Dr Rondo Middleton for his helpful advice on the data interpretation; Dr Dorothy Laflamme for her helpful comments on the manuscript; and other members of the Nestlé Purina Research Center for their contribution and support.
Footnotes
Accepted: 8 April 2019
The authors are employees of Nestlé Purina Pet Care.
Funding: This work has been funded by Nestlé Purina Pet Care.
Supplementary material: The following files are available online:
Table S1: Summary of salivary Fel d1 values for group 1
Table S2: Summary of salivary Fel d1 values for group 2
Table S3: Success (%) of correctly identifying low-producer cats based on their average salivary Fel d1 level only, or combined with their SD, in relation to the number of samples collected
ORCID iD: Berenice Bastien  https://orcid.org/0000-0003-2101-2708
https://orcid.org/0000-0003-2101-2708
Ebenezer Satyaraj  https://orcid.org/0000-0002-2807-1092
https://orcid.org/0000-0002-2807-1092
References
- 1. Gronlund H, Saarne T, Gafvelin G, et al. The major cat allergen, Fel d1, in diagnosis and therapy. Int Arch Allergy Immunol 2010; 151: 265–274. [DOI] [PubMed] [Google Scholar]
- 2. Morris DO. Human allergy to environmental pet danders: a public health perspective. Vet Dermatol 2010; 21: 441–449. [DOI] [PubMed] [Google Scholar]
- 3. Kleine-Tebbe J, Kleine-Tebbe A, Jeep S, et al. Role of the major allergen (Fel d I) in patients sensitized to cat allergens. Int Arch Allergy Immunol 1993; 100: 256–262. [DOI] [PubMed] [Google Scholar]
- 4. Ukleja-Sokolowska N, Gawronska-Ukleja E, Zbikowska-Gotz M, et al. Analysis of feline and canine allergen components in patients sensitized to pets. Allergy Asthma Clin Immunol 2016; 12: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartholome K, Kissler W, Baer H, et al. Where does cat allergen 1 come from? J Allergy Clin Immunol 1985; 76: 503–506. [DOI] [PubMed] [Google Scholar]
- 6. Van Milligen FJ, Vroom TM, Aalberse RC. Presence of Felis domesticus allergen I in the cat’s salivary and lacrimal glands. Int Arch Allergy Appl Immunol 1990; 92: 375–378. [DOI] [PubMed] [Google Scholar]
- 7. De Andrade AD, Birnbaum J, Magalon C, et al. Fel d I levels in cat anal glands. Clin Exp Allergy 1996; 26: 178–180. [DOI] [PubMed] [Google Scholar]
- 8. Varga JM, Ceska M. Characterization of allergen extracts by polyacrylamide gel isoelectrofocusing and radioimmunosorbent allergen assay. II. Dog and cat allergens. Int Arch Allergy Appl Immunol 1972; 42: 438–453. [DOI] [PubMed] [Google Scholar]
- 9. Stokes CR, Turner MW. Isolation and characterization of cat allergens. Clin Allergy 1975; 5: 241–254. [DOI] [PubMed] [Google Scholar]
- 10. Brown PR, Leitermann K, Ohman JL., Jr. Distribution of cat allergen 1 in cat tissues and fluids. Int Arch Allergy Appl Immunol 1984; 74: 67–70. [DOI] [PubMed] [Google Scholar]
- 11. Morgenstern JP, Griffith IJ, Brauer AW, et al. Amino acid sequence of Fel d I, the major allergen of the domestic cat: protein sequence analysis and cDNA cloning. Proc Natl Acad Sci U S A 1991; 88: 9690–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kristensen AK, Schou C, Roepstorff P. Determination of isoforms, N-linked glycan structure and disulfide bond linkages of the major cat allergen Fel d1 by a mass spectrometric approach. Biol Chem 1997; 378: 899–908. [DOI] [PubMed] [Google Scholar]
- 13. Kaiser L, Velickovic TC, Badia-Martinez D, et al. Structural characterization of the tetrameric form of the major cat allergen Fel d1. J Mol Biol 2007; 370: 714–727. [DOI] [PubMed] [Google Scholar]
- 14. Patchett K, Lewis S, Crane J, et al. Cat allergen (Fel d1) levels on school children’s clothing and in primary school classrooms in Wellington, New Zealand. J Allergy Clin Immunol 1997; 100: 755–759. [DOI] [PubMed] [Google Scholar]
- 15. Martin IR, Wickens K, Patchett K, et al. Cat allergen levels in public places in New Zealand. N Z Med J 1998; 111: 356–358. [PubMed] [Google Scholar]
- 16. De Lucca SD, O’Meara TJ, Tovey ER. Exposure to mite and cat allergens on a range of clothing items at home and the transfer of cat allergen in the workplace. J Allergy Clin Immunol 2000; 106: 874–879. [DOI] [PubMed] [Google Scholar]
- 17. Partti-Pellinen K, Marttila O, Makinen-Kiljunen S. Occurrence of dog, cat, and mite allergens in public transport vehicles. Allergy 2000; 55: 65–68. [DOI] [PubMed] [Google Scholar]
- 18. Woodcock A, Addo-Yobo EO, Taggart SC, et al. Pet allergen levels in homes in Ghana and the United Kingdom. J Allergy Clin Immunol 2001; 108: 463–465. [DOI] [PubMed] [Google Scholar]
- 19. Munir AK, Einarsson R, Dreborg S. Variability of airborne cat allergen, Fel d1, in a public place. Indoor Air 2003; 13: 353–358. [DOI] [PubMed] [Google Scholar]
- 20. Loan R, Siebers R, Fitzharris P, et al. House dust-mite allergen and cat allergen variability within carpeted living room floors in domestic dwellings. Indoor Air 2003; 13: 92–95. [PubMed] [Google Scholar]
- 21. Bjornsdottir US, Jakobinudottir S, Runarsdottir V, et al. The effect of reducing levels of cat allergen (Fel d1) on clinical symptoms in patients with cat allergy. Ann Allergy Asthma Immunol 2003; 91: 189–194. [DOI] [PubMed] [Google Scholar]
- 22. Gore RB, Bishop S, Durrell B, et al. Air filtration units in homes with cats: can they reduce personal exposure to cat allergen? Clin Exp Allergy 2003; 33: 765–769. [DOI] [PubMed] [Google Scholar]
- 23. Macher JM, Tsai FC, Burton LE, et al. Concentrations of cat and dust-mite allergens in dust samples from 92 large US office buildings from the BASE Study. Indoor Air 2005; 15 Suppl 9: 82–88. [DOI] [PubMed] [Google Scholar]
- 24. Liccardi G, Barber D, Russo M, et al. Human hair: an unexpected source of cat allergen exposure. Int Arch Allergy Immunol 2005; 137: 141–144. [DOI] [PubMed] [Google Scholar]
- 25. Niesler A, Scigala G, Ludzen-Izbinska B. Cat (Fel d1) and dog (Can f 1) allergen levels in cars, dwellings and schools. Aerobiologia (Bologna) 2016; 32: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wentz PE, Swanson MC, Reed CE. Variability of cat-allergen shedding. J Allergy Clin Immunol 1990; 85: 94–98. [DOI] [PubMed] [Google Scholar]
- 27. Ramadour M, Birnbaum J, Magalon C, et al. Cat sex differences in major allergen production (Fel d1). J Allergy Clin Immunol 1998; 101: 282–284. [DOI] [PubMed] [Google Scholar]
- 28. Nicholas C, Wegienka G, Havstad S, et al. Influence of cat characteristics on Fel d1 levels in the home. Ann Allergy Asthma Immunol 2008; 101: 47–50. [DOI] [PubMed] [Google Scholar]
- 29. Hussain S, Bassett C, Kaplan S, et al. Correlation between the color of cat hair and severity of allergic symptoms in patients with allergic rhinitis. J Allergy Clin Immunol 2000; 105: S5. [Google Scholar]
- 30. Hypoallergenic cat breeds. www.petmd.com (2017, accessed May 6, 2019).
- 31. Lockey RF. The myth of hypoallergenic dogs (and cats). J Allergy Clin Immunol 2012; 130: 910–911. [DOI] [PubMed] [Google Scholar]
- 32. Butt A, Rashid D, Lockey RF. Do hypoallergenic cats and dogs exist? Ann Allergy Asthma Immunol 2012; 108: 74–76. [DOI] [PubMed] [Google Scholar]
- 33. Casey RA, Vandenbussche S, Bradshaw JWS, et al. Reasons for relinquishment and return of domestic cats (Felis silvestris catus) to rescue shelters in the UK. Anthrozoös 2009; 22: 347–358. [Google Scholar]
- 34. Toribio JA, Norris JM, White JD, et al. Demographics and husbandry of pet cats living in Sydney, Australia: results of cross-sectional survey of pet ownership. J Feline Med Surg 2009; 11: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carvelli A, Iacoponi F, Scaramozzino P. A cross-sectional survey to estimate the cat population and ownership profiles in a semirural area of Central Italy. Biomed Res Int 2016; 2016. DOI: 10.1155/2016/3796872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laflamme DP. Development and validation of a body condition score system for dogs. Canine Pract 1997; 22: 10–15. [Google Scholar]
- 37. Siebers R, Healy B, Holt S, et al. Fel d1 levels in domestic living rooms are not related to cat color or hair length. J Allergy Clin Immunol 2001; 108: 652–653. [DOI] [PubMed] [Google Scholar]
- 38. Poll EM, Kreitschmann-Andermahr I, Langejuergen Y, et al. Saliva collection method affects predictability of serum cortisol. Clin Chim Acta 2007; 382: 15–19. [DOI] [PubMed] [Google Scholar]
- 39. Sun P, Sherrill S, Hannah S, et al. Correlations of saliva cytokines and sIgA with DJD in dogs. J Dent Res 2013; 92 Spec Iss A: 1334. [Google Scholar]
- 40. Silva BPLD, Knackfuss FB, Labarthe N, et al. Effect of a synthetic analogue of the feline facial pheromone on salivary cortisol levels in the domestic cat. Pesq Vet Bras 2017; 37: 287–290. [Google Scholar]
- 41. Bates D, Maechler M, Bolker B. lme4. Linear mixed-effects models using S4 classes. Vienna: R Foundation for Statistical Computing, 2012. [Google Scholar]
- 42. Bienboire-Frosini C, Cozzi A, Lafont-Lecuelle C, et al. Immunological differences in the global release of the major cat allergen Fel d1 are influenced by sex and behaviour. Vet J 2012; 193: 162–167. [DOI] [PubMed] [Google Scholar]
- 43. Charpin C, Zielonka TM, Charpin D, et al. Effects of castration and testosterone on Fel d I production by sebaceous glands of male cats: II – morphometric assessment. Clin Exp Allergy 1994; 24: 1174–1178. [DOI] [PubMed] [Google Scholar]
- 44. Kelly SM, Karsh J, Marcelo J, et al. Fel d1 and Fel d4 levels in cat fur, saliva and urine. J Allergy Clin Immunol 2018; 142: 1990–1992. [DOI] [PubMed] [Google Scholar]
- 45. Liccardi G, D’Amato G, Russo M, et al. Focus on cat allergen (Fel d1): immunological and aerodynamic characteristics, modality of airway sensitization and avoidance strategies. Int Arch Allergy Immunol 2003; 132: 1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of salivary Fel d1 values for group 1 (Table S1). Summary of salivary Fel d1 values for group 2 (Table S2). Success (%) of correctly identifying low-producer cats based on their average salivary Fel d1 level only, or combined with their SD, in relation to the number of samples collected (Table S3)