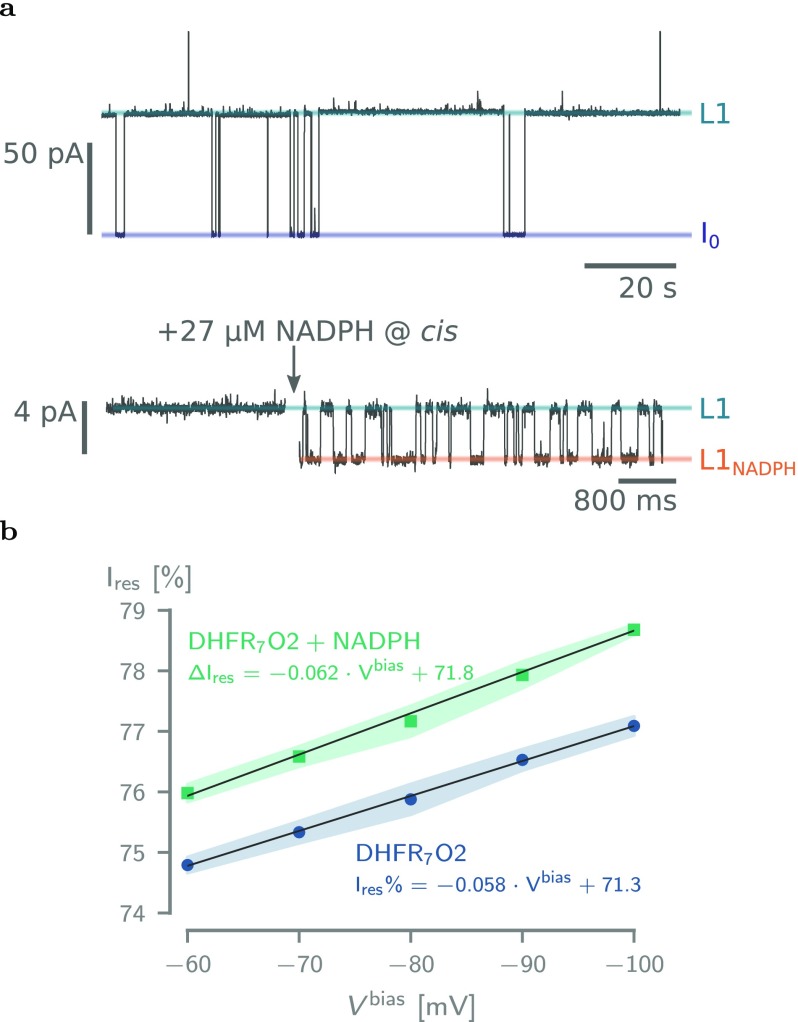
Figure 5.
Binding of NADPH to DHFR7O2. (a) Top: Typical current trace after the addition of 50 nM DHFR7O2 to a single ClyA-AS nanopore added to the cis reservoir at −60 mV applied potential. The open-pore current (I0) and the blocked pore levels (L1) are highlighted. Bottom: Current trace showing the blocked pore current of a single DHFR7O2 molecule (50 nM, cis) at −60 mV applied potential before (left) and after (right) the addition of 27 μM NADPH to the trans compartment. NADPH binding to confined DHFR molecule is reflected by current enhancements from the unbound L1 to the NADPH-bound L1NADPH current levels and showed association (kon) and dissociation (koff) rate constants of 2.03 ± 0.58 × 106 M–1·s–1 and 71.2 ± 20.4 s–1, respectively (see Table S3). (b) Dependence of the Ires% on the applied potential for DHFR7O2 and DHFR7O2 bound to NADPH. All current traces were collected in 250 mM NaCl and 15 mM Tris-HCl, pH 7.5, at 23 °C, by applying a Bessel low-pass filter with a 2 kHz cutoff and sampled at 10 kHz.