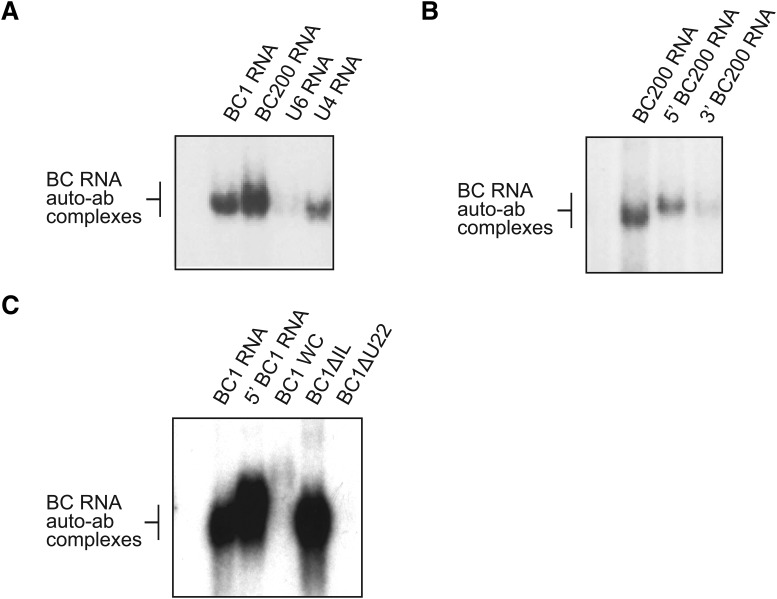
Figure 4.
Molecular targets of SLE anti-BC autoimmune reactivity. EMSA experiments were performed with 32P-labeled human BC200 RNA and rat BC1 RNA, using purified IgGs from SLE patient S6. A, Strong immune complex formation of IgG SLE S6 was apparent with BC200 RNA and BC1 RNA, with stronger reactivity toward the human than to the rat BC RNA. Immune complex formation was weak with spliceosomal U4 snRNA and undetectable with spliceosomal U6 snRNA. B, Robust reactivity was observed against full-length BC200 RNA and against the 5′ BC200 domain, but not against the 3′ BC200 domain. C, IgG SLE S6 formed immune complexes with full-length BC1 RNA and with its 5′ BC1 domain. When the 5′ DTE-resident GA motif was altered to standard A-form RNA helix by conversion of noncanonical base-pairings to standard WC base pairings (BC1 WC, see Fig. 1 for structure), full-length BC1 RNA was no longer recognized by IgG SLE S6. Similarly, IgG SLE S6 did not recognize full-length BC1 RNA in which 5′ DTE unpaired U22 had been deleted (BC1 ΔU22). By contrast, elimination of the 5′ basal internal loop (BC1 ΔIL) did not perceptibly alter BC1 RNA recognition by anti-BC IgG SLE S6. In each of the gels A-C, RNA only was loaded in the left-most lane, and no complex formation was observed. While IgG SLE S6 was used in experiments shown in this figure, analogous results were obtained with three other IgG preparations purified from highly reactive SLE sera (Table 1).