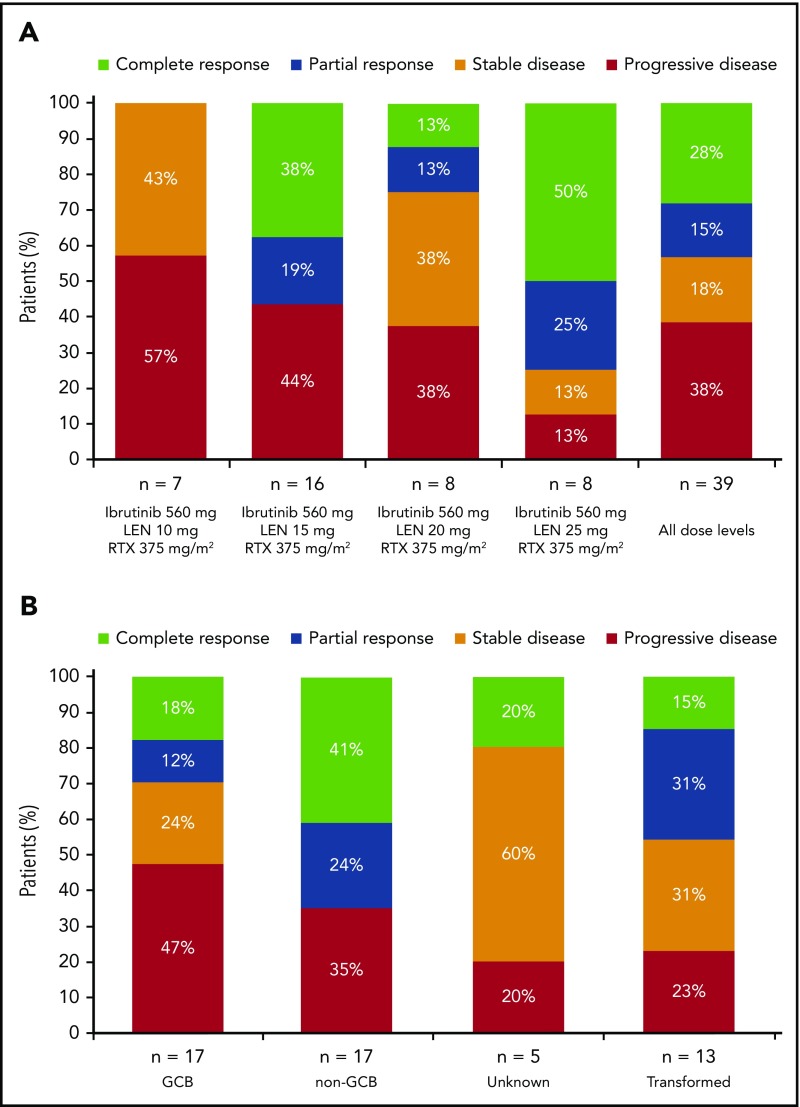
Figure 2.
Best response. Data are presented for patients in the response-evaluable population by dose cohort (A) and by DLBCL subtype/category (B). Five patients with non-GCB DLBCL treated with 15 mg lenalidomide (cohort 1, n = 3; cohort 1+, n = 2) were nonevaluable for response, and a sixth patient (20 mg lenalidomide) died of DLBCL after 5 days of treatment. The response-evaluable population included patients who had measurable disease at baseline and at least 1 posttreatment disease assessment by the investigator. LEN, lenalidomide; RTX, rituximab.