Fig. 2. Nanogel biodegradation analysis by DLS and QCM.
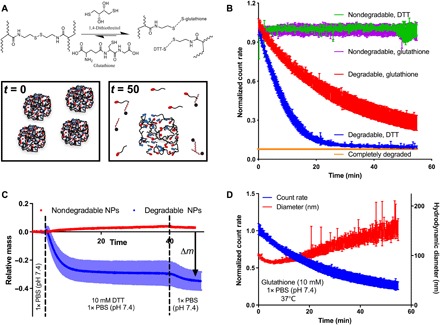
(A) N,N′-bis(acryloyl)cystamine cross-linked nanogels degrade via reduction of the disulfide. The diagram demonstrates how, after an initial period of surface erosion, the nanogels experience bulk degradation, leading to simultaneous network swelling. (B) DLS analysis of nanogel degradation. While bisacrylamide cross-linked nanogels did not degrade under reducing conditions, those cross-linked with a disulfide cross-linker were digested by both reducing agents (n = 4, mean ± SD). (C) QCM analysis demonstrated the kinetic decomposition of nanogels under reducing conditions and flow. While the mass of nondegradable nanogels was relatively unaffected by reducing conditions, the mass of degradable gels declined rapidly (n = 3, mean ± SD). (D) Hydrodynamic diameter analysis by DLS supported the degradation mechanism of initial surface erosion followed by bulk degradation. While the normalized count rate declined steadily throughout the extended measurement, the hydrodynamic diameter decreased initially (surface erosion) and then increased for the remainder of the experiment (i.e., decrease in cross-links led to a reduction in the total number of nanoparticles but swelling of the remaining intact nanogels) (n = 3, mean ± SD).
