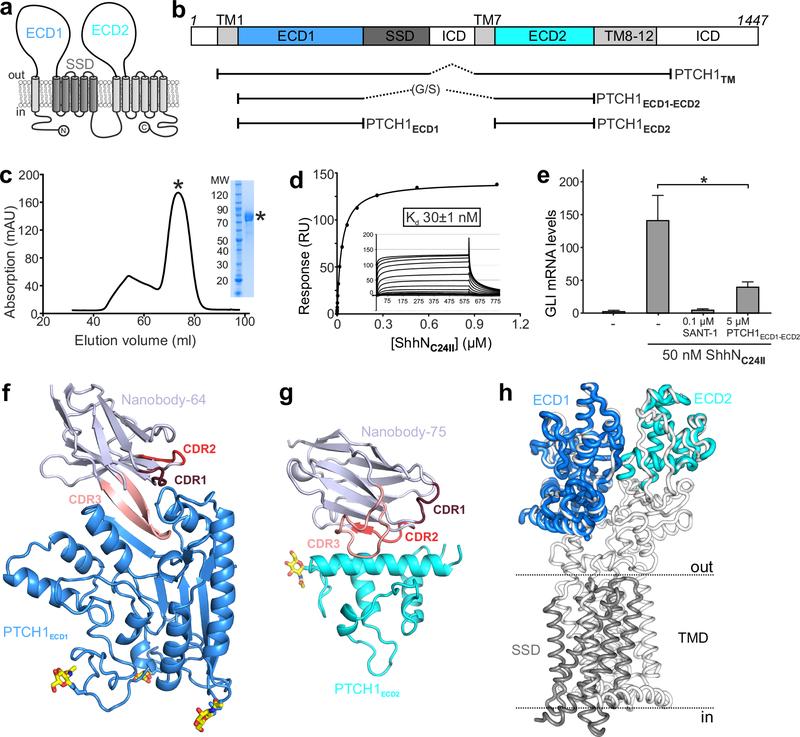
Figure 1 |. Structural and function characterization of PTCH1-nanobody interactions.
a, The pseudo-symmetric domain architecture of PTCH1: two 6-TM segments with extracellular domains (ECD1 and ECD2) interposed between the first two TM helices of each segment. The SSD, composed of TM helices 2–6, is marked in gray. b, Composition of the various protein constructs used in this study. c-e, Characterization of PTCH1ECD1-ECD2 used as an antigen to immunize Llamas. c, Typical SEC purification and corresponding SDS-PAGE of pooled fractions of PTCH1ECD1-ECD2. d, SPR equilibrium binding experiment between PTCH1ECD1-ECD2 (ligand) and non-lipidated ShhNC24II (analyte). This experiment was independently repeated 3 times with similar results. e, SHH signalling assay in mouse NIH-3T3 cells. Normalized Gli1 mRNA expression was used to assess SHH signalling activity by RT-qPCR after stimulation with purified ShhNC24II. Error bars denote SEM of 3 independent experiments. Statistical value is p=0.0387 determined by ordinary one-way ANOVA with Turkey’s multiple comparisons test. Sant-1 is a HH signalling inhibitor acting downstream of PTCH1. PTCH1ECD1-ECD2 acts as a ligand trap to inhibit Hh signalling. f-g, Cartoon representations of the high-resolution crystal structures of the PTCH1ECD1-NB64 (f) and PTCH1ECD2-NB75 (g) complexes. The complementary determining regions (CDRs) of the nanobodies are highlighted and N-linked glycans are shown in atomic colouring (carbon: yellow, oxygen: red, nitrogen: blue). h, Superposition of the PTCH1ECD1 and PTCH1ECD2 crystal structures on the previously determined cryo-EM PTCH1TM structure (PDB 6E1H16).