Abstract
Significance: Alterations in oxidant/antioxidant balance injure pulmonary endothelial cells and are important in the pathogenesis of lung diseases, such as Acute Respiratory Distress Syndrome (ARDS), ischemia/reperfusion injury, pulmonary arterial hypertension (PAH), and emphysema.
Recent Advances: The endosomal and autophagic pathways regulate cell homeostasis. Both pathways support recycling or degradation of macromolecules or organelles, targeted to endosomes or lysosomes, respectively. Thus, both processes promote cell survival. However, with environmental stress or injury, imbalance in endosomal and autophagic pathways may enhance macromolecular or organelle degradation, diminish biosynthetic processes, and cause cell death.
Critical Issues: While the role of autophagy in cellular homeostasis in pulmonary disease has been investigated, the role of the endosome in the lung vasculature is less known. Furthermore, autophagy can either decrease or exacerbate endothelial injury, depending upon inciting insult and disease process.
Future Directions: Diseases affecting the pulmonary endothelium, such as emphysema, ARDS, and PAH, are linked to altered endosomal or autophagic processing, leading to enhanced degradation of macromolecules and potential cell death. Efforts to target this imbalance have yielded limited success as treatments for lung injuries, which may be due to the complexity of both processes. It is possible that endosomal trafficking proteins, such as Rab GTPases and late endosomal/lysosomal adaptor, MAPK and MTOR activator 1, may be novel therapeutic targets. While endocytosis or autophagy have been linked to improved function of the pulmonary endothelium in vitro and in vivo, further studies are needed to identify targets for modulating cellular homeostasis in the lung.
Keywords: autophagy, endocytosis, endothelium, lung, pulmonary
Endosome Transport and Autophagy
The endosomal and autophagic pathways regulate movement of macromolecules within the cell with the primary goal of maintaining cellular homeostasis. Cells are constantly responding to intracellular and extracellular stresses and signals. In settings of increased stress or signaling, the balance of the processes may become tilted, resulting in enhanced macromolecular degradation, diminished biosynthetic processes, and possible cell death. However, the overall purpose of both is to promote cell homeostasis.
Endocytosis is the active process of moving macromolecules and particles from the environment or external membrane into the cell via invagination of the plasma membrane through one of four methods: (i) clatherin-mediated endocytosis (CME), (ii) clatherin-independent endocytosis (CIE), (iii) macropinocytosis, or (iv) phagocytosis (80, 98). CME is dependent on dynamin for membrane scission, whereas CIE can be dynamin dependent (caveolea or Rho-mediated) or independent (cdc42, Arf6, or flotillin) (80, 98). CME is a selective mechanism to internalize surface proteins, whereas CIE is believed to be more of a bulk method of endocytosis.
Macropinocytosis and phagocytosis are endocytic processes driven by an actin ring formed beneath the plasma membrane engulfing either fluid droplets or solid structures, respectively. Macropinocytosis nonselectively takes up fluid, whereas phagocytosis is initiated by the interaction of particles (>0.5 μm) to be engulfed with cell receptors, including Fcγ, C-type lectin, integrins, and scavenger receptors (22). Both processes utilize small GTPases, such as cdc42, Rac, and Rabs, to mediate the actin polymerization and endocytosis, respectively (19). Once endocytosed, the vesicular contents are sorted and can be recycled back to the cell surface, enter into the endoplasmic reticulum (ER) and Golgi complex for post-translational modification of the cargo proteins, or targeted for lysosomal degradation, autophagy, or secreted into the cytoplasm (68) (Fig. 1).
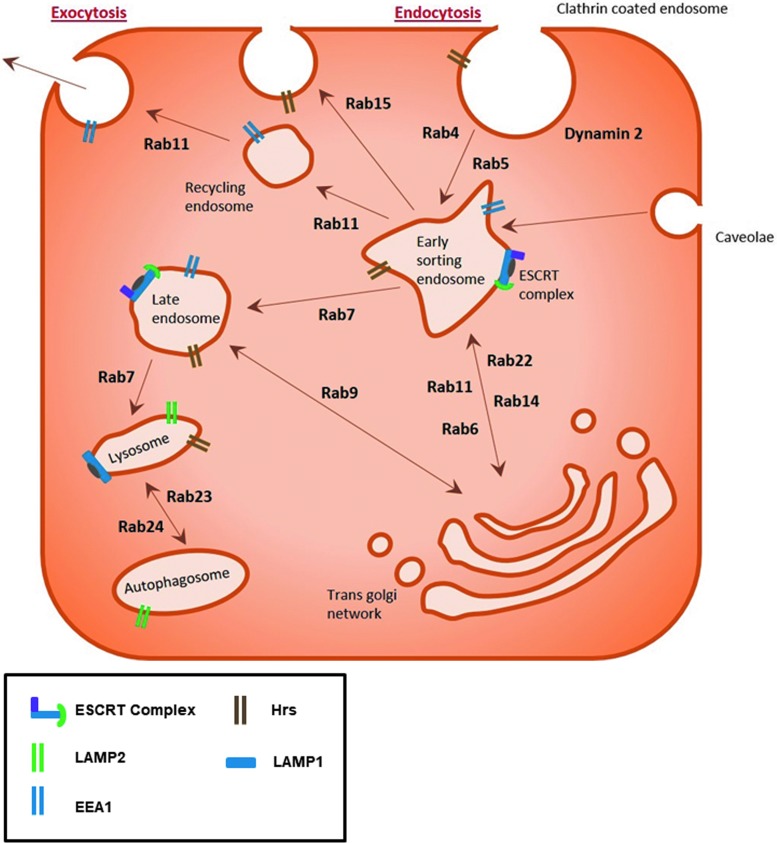
FIG. 1.
Schematic of endosome trafficking. Trafficking of the endosome through the cell is regulated through a variety of specific proteins. The process of endocytosis is mediated by invagination of the plasma membrane at either clathrin-coated regions or caveolae. Once endocytosed, cargo is sorted in the early endosome before being either recycled back to the cell surface or trafficked to the late endosome for lysosomal degradation. The spatiotemporal control of this dynamic trafficking system is regulated by a range of protein families such as Rab GTPases and ESCRTs. [Adapted from Pyrzynska et al. (69), and Zhen and Stenmark (114)]. EEA1, early endosome antigen 1; ESCRTs, endosomal sorting complexes required for transports; LAMP, lysosomal-associated membrane protein.
Protein families such as Rab GTPases, adenosine diphosphate (ADP)-ribosylation factor (Arf) G-proteins, endosomal sorting complexes required for transports (ESCRTs), and soluble N-ethylamide-sensitive factor attachment protein receptors (SNAREs) regulate the spatiotemporal control of this dynamic endosomal trafficking system. These families regulate trafficking processes through a variety of highly specialized mechanisms. Similar to the Ras GTPase superfamily, Rab GTPases require guanine exchange factors (GEFs) to promote the guanosine diphosphate (GDP) to guanosine triphosphate (GTP) exchange, allowing the change in conformation needed to bind to the endosomal membrane and mediate trafficking along the microtubule network (114).
The majority of the 70 plus Rab GTPases are involved in endocytic processes. Rab5, and effector protein early endosome antigen 1 (EEA1), associate with clathrin-coated endosomes, internalized at the plasma membrane, to mediate trafficking to the early endosome compartment (108). Rab4 and Rab11, in turn, mediate the short-loop and long-loop recycling of endosomes back to the plasma membrane, respectively (97). Rab7 and Rab9, however, promote the formation of late endosomes of cargo sorted in the trans-Golgi network (41). Early endosomes at the cell periphery undergo fusion and fission with other endosomes to create larger endosomes within the cell to allow sorting of the macromolecular contents during trafficking.
Arf G-proteins also interact with GEFs and guanine activating proteins to regulate GDP/GTP exchange and GTP hydrolysis, respectively. Arf proteins localize throughout the cell at membrane surfaces, including the plasma, endosomal, lysosomal, and secretory membranes (17). Six Arf proteins have been identified whose functions include recruitment of proteins that coat the membrane during endocytosis to promote sorting, enzymes that modify membrane lipid content, and proteins involved in cytoskeletal tethering, scaffolding, and folding (17).
Unlike Ras, Rho, and Rab GTPases, Arf G-proteins are myristoylated, which promotes their localization to membranes. Arf1 and Arf3–5 are primarily in the ER-Golgi complexes regulating retrograde transportation from the Golgi to ER and at the trans-Golgi network. Arf6 is localized at the plasma membrane and is involved in cortical actin distribution, endosomal trafficking, and rapid endosomal recycling via interactions with the microtubule motor adaptor protein, c-Jun N-terminal kinase-interacting protein-4 (17). Arf1 and Arf6 regulate intercellular junctions, adherens, and tight junctions, through the regulation of cadherin molecules at the cell surface (51, 111).
In contrast to Rab proteins, ESCRT proteins play a significant role in the delivery of cargo to the lysosome via the generation of multivesicular bodies (MVB) (67). The ESCRT protein complex is formed at endosomes during MVB sorting by sequential recruitment of ESCRT-0, -I, and -II and the ubiquitinated cargo. The ESCRT proteins form a subdomain on the endosome, which then invaginates incorporating the ubiquinated cargo. ESCRT-III functions to bud off the intraluminal vesicles within the MVB. Once MVB fuse with lysosomes, the content of the intraluminal vesicles is degraded (9). Perturbations in any of these highly regulated pathways can result in disordered trafficking of key molecules. The ESCRT complex has been implicated as having a role in the maturation process of the autophagosome (54, 73).
SNAREs are a superfamily of proteins involved in the membrane fusion of intracellular organelles, with the exception of the mitochondria (89). For membrane fusion to occur, SNARE proteins form a four-helix bundle on the adjacent membranes complex with one R-SNARE (or vesicle (v)-SNARE) on one membrane and 3 Q-SNARE (or target cell (t)-SNARE) proteins on the other membrane (89). SNARE proteins mediate merging of the membranes in close proximity and provide energy to promote the fusion of the membranes. Adenosine triphosphate (ATP)-dependent SNARE folding drives this membrane fusion. Once fused, the SNARE proteins are unfolded via the ATPase N-ethylmaleimide-sensitive factor and synaptosomal nerve-associated protein (89). Interestingly, the membrane fusion function of SNAREs is also crucial for exocytosis, autophagosome-lysosome fusion, and autophagosome biogenesis (20, 61).
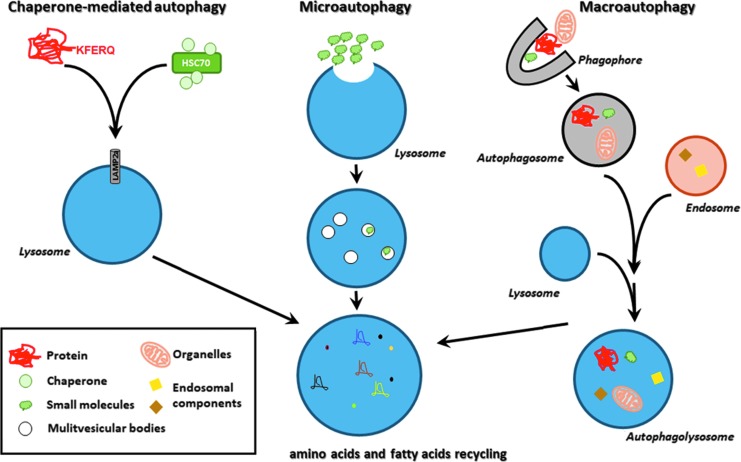
Similar to the endocytic pathway, autophagy is both a recycling and a degradative system, whereby intracellular molecules are targeted to endosomes or lysosomes, respectively. Autophagy occurs in settings of cell stress and serves to promote cell survival. In settings of excessive autophagy, cell death ensues. As described by Mizushima and Komatsu (60), there are three main types of autophagy, chaperone-mediated autophagy (CMA), microautophagy, and macroautophagy, with each resulting in the degradation of the vesicle contents (Fig. 2).
FIG. 2.
Autophagic processes. Schematic representation of chaperone-mediated autophagy, microautophagy, and macroautophagy. The three processes lead to the recycling of cellular building blocks and subsequent homeostasis. HSC70, heat shock cognate 71 kDa protein; KFERQ, pentapeptide motif (lysine-phenylalanine-glutamic acid-arginine-glutamine).
CMA is a precisely targeted event, whereby the protein cargo is recognized via a pentapeptide motif (lysine-phenylalanine-glutamic acid-arginine-glutamine) (KEFRQ)-like motif, a chaperone protein, heat shock cognate 71 kDa protein (HSC70), and its co-chaperone proteins, and subsequently internalized by a lysosomal surface receptor in conjunction with lysosomal-associated membrane protein (LAMP)2A and degraded (37). During microautophagy, subcellular small molecules enter in bulk directly into late endosomes via engulfment and, subsequently, fuse with MVB for degradation (74). Inhibition of the ESCRT complex I blocked microautophagic degradation in MVB, suggesting a role for these proteins in this process (74) and crosstalk between the endosomal and autophagic pathways.
Although initially believed to be a nonselective process, there is evidence in yeast of parallel chaperone-mediated events using HSC70 and KFERQ-like motif proteins in microautophagy and subsequent binding to phosphatidylserine on the lipid bilayer (94). HSC70 was also noted to serve as a chaperone in eukaryotic cells, which selectively targets cytosolic proteins to late endosomes that then undergo microautophagy (74). Additional chaperone proteins are believed to complex cargo proteins for microautophagy that targets select organelles, such as micromitophagy, microlipophagy, and micropexophagy, in yeast (94). The CMA process is upregulated in settings of oxidative stress due, in part, to increased LAMP2A transcription (38). Finally, macroautophagy, often just referred to as “autophagy,” is described as the most commonly occurring form of this process.
Autophagy proceeds via five main steps: initiation, nucleation, elongation, lysosome fusion, and cargo degradation. Initially, an isolation membrane or phagophore is formed from the ER, Golgi, mitochondrial, plasma, or recycling endosomal membrane. Formation of the phagophore requires altered activation and/or recruitment of a number of signaling molecules, including mammalian target of rapamycin (mTOR). In settings of cellular homeostasis, the mTOR complex 1 (mTORC1) binds to and inactivates Unc-51 like autophagy activating kinase (ULK1) or autophagy-related genes (Atg) 1 (39). On cell starvation, mTORC1 is inactivated, releasing and activating ULK1, which, in turn, binds the membranes to initiate the phagophore formation via recruitment of other proteins, including FIP200, Atg13, and Atg101 (101).
In addition to mTOR, adenosine monophosphate kinase (AMPK) has been shown to activate ULK1 directly via phosphorylation (39), as well as indirectly via Raptor-mediated inhibition of mTOR (3, 101). With the activation of ULK1, additional proteins are recruited that are necessary for the phagophore elongation. Beclin 1, originally identified as having antiapoptotic properties through its binding to B cell lymphoma 2, is a necessary component in autophagy via the formation of complex with class III-type phosphatidylinositol 3 kinase complex (29, 52).
Unlike other vesicles, phagophore double membranes are formed de novo through the synthesis of phosphatidylinositol-3-phosphate and the recruitment of microtubule-associated protein-1 light chain-3 (LC3) or Atg 8 and the Atg12-Atg5-Atg16 complex. Through a series of post-translational modifications, Atg8 is covalently attached to phosphatidylethanolamine (PE) of the lipid bilayer. Atg8-PE conjugation on the outer layer of the autophagosome is believed to regulate the size of the compartment, whereas that on the inner surface serves in the selection of cargo that is internalized (29, 32). Once the phagophore matures into the autophagosome, the outer membrane fuses with lysosomes with the release of the inner-membrane bound cargo, termed autophagic body, which is subsequently degraded, and the primary cell building blocks, such as amino acids and fatty acids, are recycled back to the cytosol (32).
In settings of excessive autophagy, cells may undergo autophagic or apoptotic cell death. Autophagy-dependent cell death occurs in settings of autophagic degradation of (i) ferritin (ferroptosis) (33) or (ii) protein tyrosine phosphatase, non-receptor type 13 (FAS-driven extrinsic apoptosis) (24); (iii) via necroptosis by utilizing the necrosome scaffolding complexes in autophagy or by cellular inhibitor of apoptosis protein (cIAP)-1 and cIAP-2 degradation (23); and (iv) by autosis, which is mediated via Na+/K+ ATPase (48). Apoptosis or programmed cell death is a well-studied regulated cell death (RCD) process that may occur by signals that are initiated intrinsically or extrinsically and can occur in a caspase-independent manner. More in-depth reviews on apoptosis are available (27, 85).
Regulated endosomal trafficking is key for efficient autophagy; indeed, the autophagosome membrane has been demonstrated to originate from the endosomal membrane (57). Maturation of the autophagosome, by fusion with the lysosome, forms the autophagolysosome where autophagy can occur. This fusion process allows the delivery of key lysosomal components such as hydrolases to degrade autophagocytosed products, the H+-vacuolar ATPase to acidify the vesicle, and transporters and permeases needed to allow efficient recycling of autophagic degradation products (82).
Endosomal proteins form the molecular machinery required for formation of the autophagosome. For example, several Rab GTPases (Rab11, Rab33b, Rab5, and Rab7) as well as the EEA1 have all been demonstrated to impact autophagy by using in vitro studies (8, 31, 87, 100). Several of these trafficking molecules regulate dynamics of the recycling endosome to regulate fusion between MVB and the autophagosome (Rab11), push the recycling endosome into the late endosome/lysosome pathway (Rab7), or promote trafficking of endosomes from the Golgi to the maturing autophagosome (Rab33b) (49, 88, 90). These proteins sort endosomes early in the trafficking pathway, promoting the formation of the lysosome and subsequent maturation into the autophagolysosome, which enables autophagic degradation of targeted cargo.
In addition to LC3/Atg5/Atg7-mediated autophagic flux, studies also indicate the presence of an independently mediated autophagosome formation pathway (65). This LC3/Atg5/Atg7-independent autophagy is linked to the fusion of Rab9-positive endosomes with the autophagosome. Interestingly, both Rab7 and Rab9 GTPase are also involved in autophagosomal membrane formation, which occurs during mitophagy (30, 103). Recent papers suggest that Rab5 mediates the closure of the autophagosome before fusing with lysosomes in yeast cells (10, 115). More recently, data demonstrate that Rab5 recruits ESCRT to complete the autophagosomal scission (115). It is clear that there is crosstalk between the endocytic and autophagic processes and therefore likely that Rab GTPases mediate these two key pathways, which control cellular homeostasis.
The master regulator of the lysosome, mTORC1, also plays a key role in autophagy. In nutrient-rich settings, mTORC1 reduces autophagy by inhibiting catabolic pathways, in close coordination with AMPK, and promotes biosynthesis pathways to increase cell growth (78). mTORC1 responds to several upstream stress signals, such as amino acids and growth factors, via H+-vacuolar ATPase and Rheb GTPase localized to the lysosome surface (77, 84). Amino acid signals from the lumen of the lysosome to the Ragulator complex result in the recruitment of mTORC1 to the lysosome (70). The Ragulator complex has, therefore, been the focal point of much autophagy research, with studies indicating that the H+-vacuolar ATPase-Ragulator interaction activates the GTP-loading of Rag GTPases, leading to mTORC1 recruitment, its activation by Rheb, and the subsequent inhibition of autophagy (77).
There are a range of proteins that comprise the Ragulator complex, including GTPases RagA-RagD and scaffold proteins late endosomal/lysosomal adaptor, MAPK and MTOR activator 1–5 (62, 77). LAMTOR1 provides the scaffold for Rag GTPases to recruit mTORC1, and the LAMTOR2/3 and LAMTOR4/5 heterodimers anchor the complex to the lysosome membrane (63). As such, inhibition of LAMTOR1 in mouse embryonic fibroblasts has been demonstrated to promote autophagy, through decreased mTOR phosphorylation and increased LC3II formation, in settings of nutrient deprivation (107). Interestingly, the Ragulator complex also regulates mitogen-activated protein kinase kinase signaling from the late endosome, associated with lysosome biogenesis, membrane dynamics, and organelle transport along the cytoskeleton (63). Members of the Ragulator complex, such as LAMTORs, therefore represent interesting molecular targets to modulate autophagy in settings of disease.
Function of Endocytosis and Autophagy in Healthy Endothelium
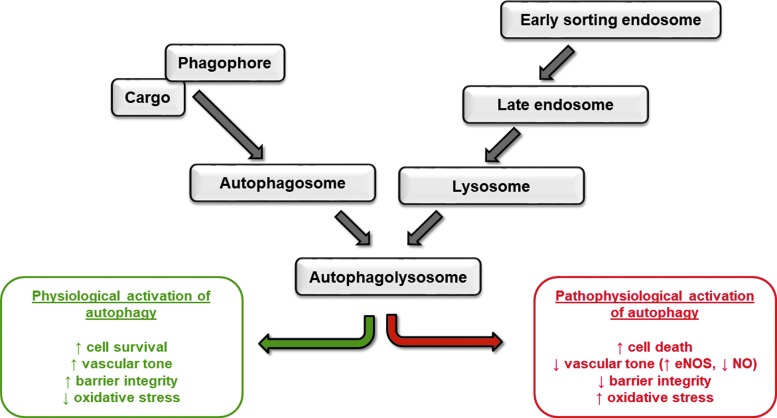
The flow of macromolecules in the endothelial cells through endosomal and autophagic trafficking is important in regulating functional vasculature, angiogenesis, and responding to aging and changes in the environment. Inhibition of the basal levels of either process leads to endothelial cell apoptosis, permeability, migration, and vessel regression. Conversely, excessive activation of endocytosis or autophagy can exacerbate vascular injury, leading to pathological progression of diseases (Fig. 3).
FIG. 3.
Physiological and pathological regulation of pulmonary circulation by endosomal and autophagic signaling. Schematic representation of the intersection of endocytosis and autophagy and the effect on endothelial cell function. eNOS, endothelial nitric oxide synthase; NO, nitric oxide.
Endocytosis is important in the trafficking of many cell surface molecules, including adherens and tight junction proteins, receptor tyrosine kinases (RTK), as well as proteins localized near the plasma membrane, such as Rho and Rac GTPases. We have shown upregulation of recycling endosomal proteins, p18 or Rab4, to increase vascular endothelial (VE)-cadherin surface expression in pulmonary endothelial cells; protect against barrier dysfunction (11, 13); and promote migration, proliferation, and angiogenesis (11, 12, 93). The long-loop recycling Rab GTPase, Rab11a, provides the platform for LC3-positive autophagosomes to mature and has also been shown to protect the pulmonary endothelium against leak, through improved adherens junction formation (104). Other studies have similarly shown tight junction proteins, including zonula occludens, junctional adhesion molecule-A, claudins, and occludin, to be regulated by endosomal trafficking affecting endothelial barrier function [reviewed in (86)].
RTK were believed to function at the plasma membrane to regulate endothelial cell function; however, it is now recognized that several RTK instead signal from the cytosol via endosomes. For example, vascular endothelial growth factor receptor 2 (VEGFR2) and fibroblast growth factor receptor 1 are localized to endosomes. Once bound by vascular endothelial growth factor-A or -C, VEGFR2 internalization occurs via the clathrin/dynamin-mediated pathway (7), which then leads to Rab5-mediated trafficking to early endosomes and recycling via Rab11 (5) or via macropinocytosis (6). In addition, unbound VEGFR2 is noted to undergo constitutive endocytosis and recycling to the plasma membrane via Rab4 (40), as well as shear stress-induced caveolin-dependent endocytosis of VEGFR2.
Interendothelial cell junctions are transiently disrupted by some edemagenic agents, such as thrombin, via actions of Rho A and Rac 1. In settings of low levels of Rho B or deficiency, Rac 1 is recycled via endosomes to the plasma membrane promoting barrier function. It has been shown that endosomal Rho B diminishes Rac 1 plasma localization by targeting it to late endosomes for degradation, causing delayed barrier recovery (56). As Rac 1 is involved in other endothelial cell functions, such as migration, it is likely that this molecule is also impacted by endosomal trafficking.
Autophagy has been shown to regulate the levels of nitric oxide (NO) in settings of increased shear stress on static endothelial cell monolayers and ex vivo perfusion of the carotid artery (25) and in aging (44). The changes in shear stress correlated with increased levels of p62, beclin and LC3, autophagy markers; increased endothelial nitric oxide synthase (eNOS); showed a reduction in endothelin-1 (25); and increased Rab4 expression (106). Beclin and p62 were also reduced in the brachial artery endothelial cells of older relative to younger human subjects and mice, with a corresponding decrease in endothelial-dependent dilation and NO bioavailability with an increase in oxidative stress and inflammation (44). Further, in settings of high shear stress, autophagy was required to promote endothelial cell alignment with the flow, protect against endothelial cell apoptosis or senescence, and plaque formation (99).
Similarly, autophagy attenuates oxidant injury to endothelial cells. Ha and colleagues showed that curcumin induced autophagy in human umbilical vein endothelial cells exposed to hydrogen peroxide via increased LC3II and number of autophagosomes to promote cell viability (26). After cigarette smoke exposure, elevated autophagy and senescence have been observed in endothelial cells and lungs, respectively (Fig. 4).
FIG. 4.
Cigarette smoke exposure increases markers of autophagy in pulmonary endothelial cells. Pulmonary microvasculature endothelial cells were transfected with ptfLC3, which encodes LC3 fused to both RFP and GFP proteins; visualization via fluorescence microscopy of autophagic vacuoles (RFP+/GFP+; RFP+/GFP−), autophagosomes (RFP+/GFP+), and autolysosomes (RFP+/GFP−). The endothelial cells were exposed to vehicle (a, d), CSE (b, d) for 2 h, or serum deprivation as a positive control for 30 min (c). (a–c) Immunofluorescence of RFP and GFP was used to assess the level of RFP-positive autophagic vesicles. Yellow arrows indicate co-expression of RFP and GFP that is indicative of autophagosomes, whereas white arrows show RFP staining that is indicative of autophagolysosomes. (d) Expression of LC3I and II was assessed by Western blotting, with increased LC3II indicative of autophagy. (H.C. and E.O.H; Unpublished data). CSE, cigarette smoke extract; GFP, green fluorescent protein; LC3, light chain-3; RFP, red fluorescent protein.
Other studies have shown that induction of autophagy prevented against stroke by diminishing reactive oxygen species (ROS) and improving vascular relaxation of the carotid (43), improved NO signaling in endothelial cells isolated from patients with type 2 diabetes (21), and improved endothelial-dependent dilation in aged animals (44). Rab26, a pro-autophagic GTPase that targets activated Src to LC3-positive autophagosomes, protects the pulmonary endothelial barrier by stabilizing adherens junctions (18, 46). Rather confusingly, Rab5a and Rab9, implicated as pro-autophagic GTPases, negatively regulate barrier function in the vasculature with overexpression of the dominant-negative, GDP-locked Rab9 mutant in the pulmonary endothelium attenuating bacteria- and endotoxin-induced barrier disruption both in vitro and in vivo (11, 105). These and other studies suggest that autophagy is necessary for proper vascular response and disruption of autophagy can lead to a dysfunctional endothelium.
Endosomal Signaling in the Pulmonary Endothelium in Lung Diseases
Acute respiratory distress syndrome
Although studies demonstrate a role of the endosome in regulating the endothelium, less is known about the role in the lung. We and others have demonstrated that members of the endosomal cycling pathways, Rab GTPase family, Rab4, Rab5, Rab9, and Rab11, play a key role in regulating microvascular permeability and edema formation in both in vitro and in vivo models of acute respiratory distress syndrome (ARDS) (11, 104, 105). Activation of the pro-recycling GTPases, Rab4, and Rab11, and inhibition of pro-degradative GTPase, Rab9, elevates trafficking of the junctional protein, VE-cadherin, to the adherens junction, thereby improving barrier integrity and reducing lipopolysaccharide (LPS)- and thrombin-induced vascular leak (Fig. 5) (11, 104). Conversely, inhibition of GTP-bound Rab5, the GTPase associated with endosome internalization, attenuated LPS-induced barrier disruption (105), indicating the importance of this small GTPase family in regulating the pulmonary microvasculature.
FIG. 5.
Endosome regulators are both vascular protective and vascular disruptive. (a) Pulmonary microvascular endothelial cells were transiently transfected with dominant active Rab4 (Rab4Q67L), dominant negative Rab9 (Rab9S21N), Rab7 (Rab7T22N) constructs, or a GFP vector control, and they were exposed to LPS for 6 h. (a), (i) whole-cell ELISA was used to assess the cell-surface expression of the adherens junction protein, VE-cadherin and (ii) electric cell impedance system was used to measure the permeability as the drop in endothelial monolayer resistance. n = 6, *p < 0.05 versus vehicle for LPS [adapted from Chichger et al. (11)]. (b) A schematic figure representing the key vascular protective and vascular disruptive endosomal regulators. Arf, ADP-ribosylation factor; CLIC4, chloride intracellular channel 4; ELISA, enzyme-linked immunosorbent assay; Hrs, hepatocyte growth factor-regulated tyrosine kinase substrate; LPS, lipopolysaccharide; VE, vascular endothelial.
Pulmonary hypertension
Recent studies have shown expression of the chloride intracellular channel 4 (CLIC4) to be upregulated in the lungs of pulmonary arterial hypertension (PAH) patients and rodents (102) and in endothelial colony-forming units from PAH patients (1). Likewise, Arf6 was also increased in endothelial colony-forming units from PAH patients (1). CLIC4 localizes to endocytic vesicles, where it interacts with Arf G-proteins triggering the acidification of the vesicles to lysosome and targeting bone morphogenetic protein receptor II (BMPRII) for degradation (1). As such, CLIC4 was identified to regulate pulmonary endothelial cell function (81, 96), and targeted disruption of CLIC4 or Arf6 expression was identified to attenuate PAH (1).
Other studies have shown that the vasomotor tone of the pulmonary vasculature is regulated via endocytosis of eNOS in vivo. Caveolin-1 binding of eNOS in the caveolae serves to inhibit its function (58). On endocytosis, caveolin-1 is phosphorylated, releasing eNOS from an inhibitory state promoting the synthesis of NO (58). The vasodilatory response pulmonary vasculature in caveolin null mice is attenuated (53). Heritable mutations of caveolin-1 predispose individuals to developing PAH (4). A recent study identified a frameshift mutation in the carboxyl terminus of caveolin-1; it disrupts its retention in the ER, resulting in its degradation and diminished caveolae formation (15). Thus, it is likely that the development of pulmonary hypertension (PH) is due, in part, to disrupted endocytosis of surface proteins, such as BMPRII, eNOS, or other proteins that are important in regulating the pulmonary vasculature.
Autophagic Signaling in the Pulmonary Endothelium in Lung Diseases
Recent reviews have summarized evidence for RCD and autophagy in lung diseases (2, 59, 79). In many lung diseases, oxidative stress alters protein folding, revealing amino acid motifs that are subsequently recognized by CMA machinery and targeted for autophagy, thereby restoring homeostasis. In addition, mitochondrial injury may result in mitophagy and removal of damaged organelles. However, unchecked autophagy can also have deleterious effects, including excessive apoptosis and impaired angiogenesis.
Table 1 summarizes the effects of various lung injuries on autophagy in lungs and endothelial cells and the effects of autophagy on function. In general, autophagic processes are identified in lungs and cultured cells by increased expression of autophagy markers, such as increased ratio of microtubule-associated protein 1 LC3-II to LC3-I and characteristic autophagosome morphology detected by transmission electron microscopy. Effects of autophagy on cell or organ function are generally inferred by the functional effects of autophagy inhibitors (e.g., 3-methyladenine [3-MA]), activators (e.g., rapamycin), or by altering the expression of autophagy regulators (e.g., early growth response-1 [Egr-1]).
Table 1.
Autophagy in Lung Diseases
| Lung disease | Cause | Autophagy marker in lung | Autophagy marker in lung endothelial cells | Effect of autophagy on function | Reference |
|---|---|---|---|---|---|
| PH | Idiopathic PAH | ↑ | ND | Improve | (92) |
| Hypoxia | ↑ | ↑ | Improve | (92) | |
| Sugen-hypoxia | ↑ | ↑ | Improve | (93) | |
| Methamphetamine | ND | ↑ | Improve | (94) | |
| Persistent PH of newborn | ↑ | ↑ | Worsen | (95) | |
| HIV-tat/morphine | ↑ | ↑ | ND | (96) | |
| Hypoxia/Ucp2 knockout | Mitophagy ↑ | ↑ | Worsen | (97) | |
| ARDS | Bacterial pneumonia | Xenophagy ↑ | ND | Improve | (99) |
| Sepsis | ND | ND | Improve | (100) | |
| Hyperoxia | ↓ | ↓ | Worsen | (101) | |
| H5N1 infection | ↑ | ND | Worsen | (102) | |
| Endotoxin | ND | ↑ | Improve | (103) | |
| Endotoxin | ND | ND | Improve | (104) | |
| Cigarette smoke | ND | ↑ | ND | (113) | |
| Ischemia/reperfusion | PA occlusion | ↑ | ND | Worsen | (107) |
| Lung transplant | Mitophagy ↑ | ↑ | Worsen | (106) | |
| Emphysema | Cigarette smoke | Mitophagy ↑ | ND | Worsen | (88) |
| Cigarette smoke | Ciliophagy ↑ | ND | Worsen | (110) | |
| Cadmium | ND | ↑ | Improve | (114) |
Summary of literature demonstrating autophagy, including endothelial cell autophagy, has been demonstrated in lungs after various insults.
ARDS, acute respiratory distress syndrome; ND, not done; PA, pulmonary arterial; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension.
Pulmonary hypertension
PH is characterized by vascular remodeling with abnormal proliferation of vascular cells, including endothelial cells, with increased intimal thickness and formation of plexiform lesions in PAH. Studies in animal models have demonstrated initial endothelial apoptosis, followed by proliferation of apoptosis-resistant endothelial cells (75). Autophagy has been demonstrated in lungs of humans with PAH and may serve a protective role against endothelial injury that initiates vascular remodeling in PH [reviewed in (109)].
Lee et al. (45) demonstrated increased expression of the autophagy marker, LC3B-II, in the endothelium of lungs from humans with PAH, with increased LC3B-II protein levels in lung tissue from PAH (IPAH) versus non-PH controls, and in hypoxia-induced PH in mice. Genetic downregulation of LC3B-II or Egr-1 (transcriptional regulator of LC3B-II) resulted in exacerbation of PH in mice and increased hypoxia-induced proliferation of endothelial and smooth muscle cells, suggesting that autophagy keeps vascular cell growth in check and may protect against PH development. Similarly, using the Sugen/hypoxia model of severe PH in rats, Kato et al. (36) demonstrated increased expression of autophagy markers LC3 and beclin-1 in parallel with worsening PH and endothelial cell proliferation.
Rapamycin, an activator of autophagy, decreased PH and endothelial cell proliferation, with a concomitant increase in endothelial cell autophagy and apoptosis, suggesting that endothelial cell autophagy suppressed the progression of endothelial cell proliferation, resulting in diminished PH (36). Indeed, this is the case in methamphetamine-associated PAH, which occurs in individuals with a single nucelotide polymorphism that reduces expression of the methamphetamine metabolizing enzyme, carboxylesterase 1 (66). Pulmonary microvascular endothelial cells that are deficient in carboxylesterase 1 demonstrated reduced autophagic flux and increased apoptosis in response to methamphetamine. These results suggest that autophagy is protective against methamphetamine-induced PAH.
However, others have demonstrated deleterious effects of autophagy in PH. Persistent PH of the newborn is characterized by decreased lung blood vessel density. Teng et al. (95) reported increased expression of autophagy markers in pulmonary artery endothelial cells isolated from lungs of lambs with persistent PH of the newborn. Inhibition of autophagy increased in vitro angiogenesis, suggesting that autophagy impairs angiogenesis in persistent PH of the newborn.
In humans with HIV infection and in a simian model of HIV-associated PAH exposed to morphine, Dalvi et al. (16) observed hyperproliferative and apoptosis-resistant endothelium that expressed autophagy markers. Since opioid use increases the risk of developing PH in HIV-infected individuals, the authors assessed the effects of HIV-tat and morphine on cultured pulmonary endothelial cells. They noted increased autophagy flux and apoptosis in human pulmonary microvascular endothelial cells when treated with HIV-tat protein and morphine in an ROS-dependent manner. Autophagy inhibitors increased and activators diminished apoptosis. In addition, HIV-tat plus morphine-induced increases in ROS were regulated by autophagy and autophagy inhibition increased ROS, suggesting that autophagy is regulating ROS. They concluded that autophagy of pulmonary endothelial cells may increase the extent of angioproliferative remodeling and thus the severity of HIV-associated PH.
Similarly, Haslip et al. (28) studied a mouse model of PH caused by intermittent hypoxia. They found that mice deficient in mitochondrial uncoupling protein 2 (UCP2) had more severe PH after 5 weeks of intermittent hypoxia. Mouse lung endothelial cells deficient in UCP2 demonstrated increased mitophagy and apoptosis, similar to human pulmonary artery endothelial cells from humans with PAH. They also concluded that endothelial cell autophagy was deleterious in PH. Mao et al. (55) demonstrated that hypoxia-induced pulmonary vascular angiogenesis is dependent on increased autophagy.
Thus, whether autophagy is protective or deleterious in PH depends on whether it limits or increases abnormal endothelial proliferation or whether it prevents normal angiogenesis. More work is needed to determine the impact of autophagy on the pulmonary endothelial function in the setting of increased ROS and PH.
Acute respiratory distress syndrome
ARDS is a syndrome of pulmonary edema, hypoxemia, and low lung compliance, frequently associated with inflammation and increased oxidant stress. Edema in ARDS is caused by increased pulmonary microvascular permeability, resulting in water and protein flux out of the vasculature and into the lung interstitium and alveolar gas space. Injury to the lung endothelium and epithelium are critical to increased permeability edema in ARDS.
Autophagy can be protective in ARDS caused by bacterial pneumonia by facilitating removal of pathogens by macrophages (xenophagy) (72). Similarly, autophagy can protect against sepsis-induced mitochondrial damage and downstream inflammasome activation and cytokine release, demonstrating a role for autophagy in regulating innate immune responses (64).
Hyperoxia (95% oxygen for 48–72 h) is a mouse model of severe oxidant lung injury characterized by inflammation, increased vascular permeability, and endothelial cell apoptosis. Silencing of lung endothelial hemoxygenase-1 (HO-1) increased inflammation, worsened survival, and increased endothelial apoptosis after hyperoxic exposure (113). Hyperoxia decreased expression of autophagy markers in both mouse lungs and cultured lung endothelial cells, an effect that was exacerbated by HO-1 suppression. These results indicate that hyperoxia decreases endothelial autophagy and suggest that HO-1 modulates this protective mechanism.
On the other hand, the lungs of humans and mice with H5N1 influenza infection demonstrated accumulation of autophagosomes and increased the ratio of LC3B-II:LC3B-I autophagy marker expression (91). Studies of alveolar epithelial cells showed that H5N1 virus, but not H1N1, induced autophagy and decreased viability. Inhibition of autophagy by silencing Atg5 or by 3-MA prolonged survival and decreased edema resulting from H5N1 pulmonary infection. Thus, in the case of lung injury caused by H5N1 viral pneumonia, autophagy in epithelial cells appears to be deleterious.
Similarly, recent studies indicate that autophagy enhanced LPS-induced endothelial barrier dysfunction. Zhang et al. (110) reported that a chemical inhibitor of autophagy, chloroquine, and silencing of Atg7 blunted LPS-induced autophagosome formation in human pulmonary microvascular endothelial cells, decreased cell viability, and exacerbated LPS-induced monolayer permeability. Similarly, Slavin et al. (83) reported that the autophagy inhibitor, 3-MA, reduced inhaled LPS-induced lung edema both when given prophylactically and as a treatment with minimal effects on inflammation and proinflammatory cytokine expression. In isolated endothelial cells, 3-MA or silencing of Atg5 blunted thrombin-induced barrier dysfunction and reversed LPS-induced endothelial barrier dysfunction. Thus, autophagy appears to play a key role in promoting the breakdown of the endothelial barrier in response to edemagenic agents.
The role(s) of autophagy in acute lung injuries resulting in ARDS are, therefore, complex and dependent on cell type studied and cause of lung injury. It has been proposed that therapeutic approaches to ARDS should not focus on autophagy alone, but on autophagy plus protection against oxidative stress, such as that conferred by Nrf2, a transcription factor that promotes genes that regulate oxidative stress (71).
Ischemia/reperfusion lung injury
Ischemia/reperfusion lung injury can occur with pulmonary thromboembolectomy and lung transplantation and is characterized by lung inflammation and edema. Autophagy markers were increased in rodent lung models of ischemia/reperfusion injury (47, 112) Inhibition of autophagy by 3-MA alleviated and rapamycin activation of autophagy aggravated lung injury (47, 112). Pulmonary microvascular endothelial cells exposed to hypoxia, followed by reoxygenation, displayed increased mitophagy and apoptosis (47). These results suggest a role for autophagy in endothelial injury caused by ischemia/reperfusion.
Emphysema
Emphysema is characterized by loss of alveolar/capillary septum and increased apoptosis of epithelial and endothelial cells (34, 35). Damaged organelles, such as mitochondria (59) and cilia (42), can be disposed off through autophagy (14) in chronic obstructive pulmonary disease. Mitophagy and ciliophagy have been demonstrated in bronchial epithelial cells. It is not clear to what extent mitophagy occurs in lung microvascular endothelial cells in emphysema.
With regard to the lung endothelium, we have reported that acute exposure to cigarette smoke increased lung vascular permeability and endothelial monolayer permeability through an oxidant-mediated effect (50). We also found that cigarette smoke extract increased cultured lung endothelial cell apoptosis and expression of markers of the unfolded protein response (eIF2a phosphorylation) and autophagy (the increased ratio of LC3B-II:LC3B-I) (76).
Using a mouse model of emphysema caused by intratracheal instillation of cadmium, Surolia et al. (92) demonstrated worsening of emphysema in HO-1 knockout mice. Exposure of lung endothelial cells to cadmium increased both apoptosis and expression of autophagy markers. Cadmium exposure of endothelial cells from HO-1 knockout mice increased apoptosis and decreased autophagy; whereas overexpression of HO-1 or treatment with the autophagy activator, rapamycin, decreased apoptosis and increased autophagy. These results indicate that HO-1 induces protective autophagy in lung endothelial cells and is protective against cadmium-induced emphysema.
Table 1 demonstrates that autophagy, including endothelial cell autophagy, has been demonstrated in lungs after various insults. However, the effects of autophagy on lung or endothelial cell function—usually assessed by the effects of autophagy inhibitors or activators or by suppression of key autophagy effectors—vary among injuries. Thus, there is a need for additional study of the role of autophagy in lung diseases.
Acknowledgments
This work was supported by Diabetes United Kingdom grant 15/0005284 (H.C.), RO1 HL123965 (E.O.H.), VA Merit Review (S.R.), U54 GM115677 (S.R.), and P20 GM103652 (E.O.H. and S.R.). Some of the research reported in this article was supported with the use of facilities at the Providence VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Abbreviations Used
- 3-MA
3-methyladenine
- ADP
adenosine diphosphate
- AMPK
adenosine monophosphate kinase
- ARDS
acute respiratory distress syndrome
- Arf
ADP-ribosylation factor
- Atg
autophagy-related genes
- BMPRII
bone morphogenetic protein receptor II
- cIAP
cellular inhibitor of apoptosis protein
- CIE
clatherin-independent endocytosis
- CLIC4
chloride intracellular channel 4
- CMA
chaperone-mediated autophagy
- CME
clatherin-mediated endocytosis
- EEA1
early endosome antigen 1
- Egr-1
early growth response-1
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ESCRTs
endosomal sorting complexes required for transports
- GDP
guanosine diphosphate
- GEF
guanine exchange factors
- GFP
green fluorescent protein
- GTP
guanosine triphosphate
- HO-1
hemoxygenase-1
- HSC70
heat shock cognate 71 kDa protein
- LAMP
lysosomal-associated membrane protein
- LC3
light chain-3
- LPS
lipopolysaccharide
- mTORC1
mTOR complex 1
- mTOR
mammalian target of rapamycin
- MVB
multivesicular bodies
- NO
nitric oxide
- PAH
pulmonary arterial hypertension
- PE
phosphatidylethanolamine
- RCD
regulated cell death
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinases
- SNAREs
soluble N-ethylamide-sensitive factor attachment protein receptors
- UCP2
mitochondrial uncoupling protein 2
- ULK1
Unc-51 like autophagy activating kinase
- VE
vascular endothelial
- VEGFR2
vascular endothelial growth factor receptor 2
References
- 1. Abdul-Salam VB, Russomanno G, Chien-Nien C, Mahomed AS, Yates LA, Wilkins MR, Zhao L, Gierula M, Dubois O, Schaeper U, Endruschat J, and Wojciak-Stothard B. CLIC4/Arf6 pathway. Circ Res 124: 52–65, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aggarwal S, Mannam P, and Zhang J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 311: L433–L452, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alers S, Löffler AS, Wesselborg S, and Stork B. Role of AMPK-mTOR-ULK1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol Cell Biol 32: 2–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA, 3rd, Palomero T, Sumazin P, Kim HR, Talati MH, West J, Loyd JE, and Chung WK. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 5: 336–343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ballmer-Hofer K, Andersson AE, Ratcliffe LE, and Berger P. Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood 118: 816–826, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Basagiannis D, Zografou S, Murphy C, Fotsis T, Morbidelli L, Ziche M, Bleck C, Mercer J, and Christoforidis S. VEGF induces signalling and angiogenesis by directing VEGFR2 internalisation through macropinocytosis. J Cell Sci 129: 4091–4104, 2016 [DOI] [PubMed] [Google Scholar]
- 7. Bhattacharya R, Kang-Decker N, Hughes DA, Mukherjee P, Shah V, McNiven MA, and Mukhopadhyay D. Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J 19: 1692–1694, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Bröcker C, Engelbrecht-Vandré S, and Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol 20: R943–R952, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Campsteijn C, Vietri M, and Stenmark H. Novel ESCRT functions in cell biology: spiraling out of control? Curr Opin Cell Biol 41: 1–8, 2016 [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Zhou F, Zou S, Yu S, Li S, Li D, Song J, Li H, He Z, Hu B, Björn LO, Lipatova Z, Liang Y, Xie Z, and Segev N. A Vps21 endocytic module regulates autophagy. Mol Biol Cell 25: 3166–3177, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chichger H, Braza J, Duong H, Boni G, and Harrington EO. Select Rab GTPases regulate the pulmonary endothelium via endosomal trafficking of vascular endothelial-cadherin. Am J Respir Cell Mol Biol 54: 769–781, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chichger H, Braza J, Duong H, Stark M, and Harrington EO. Neovascularization in the pulmonary endothelium is regulated by the endosome: Rab4-mediated trafficking and p18-dependent signaling. Am J Physiol Lung Cell Mol Physiol 309: L700–L709, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chichger H, Duong H, Braza J, and Harrington EO. p18, a novel adaptor protein, regulates pulmonary endothelial barrier function via enhanced endocytic recycling of VE-cadherin. FASEB J 29: 868–881, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cloonan SM. and Choi AM. Mitochondria in lung disease. J Clin Invest 126: 809–820, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Copeland CA, Han B, Tiwari A, Austin ED, Loyd JE, West JD, and Kenworthy AK. A disease-associated frameshift mutation in caveolin-1 disrupts caveolae formation and function through introduction of a de novo ER retention signal. Mol Biol Cell 28: 3095–3111, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalvi P, Sharma H, Chinnappan M, Sanderson M, Allen J, Zeng R, Choi A, O'Brien-Ladner A, and Dhillon NK. Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: Role in HIV-related pulmonary arterial hypertension. Autophagy 12: 2420–2438, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donaldson JG. and Jackson CL. Arf family G proteins and their regulators: Roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 12: 362–375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong W, He B, Qian H, Liu Q, Wang D, Li J, Wei Z, Wang Z, Xu Z, Wu G, Qian G, and Wang G. Rab26-dependent autophagy protects adherens junctional integrity in acute lung injury. Autophagy 14: 1677–1692, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egami Y. Molecular imaging analysis of Rab GTPases in the regulation of phagocytosis and macropinocytosis. Anat Sci Int 91: 35–42, 2016 [DOI] [PubMed] [Google Scholar]
- 20. Fader CM, Sánchez DG, Mestre MB, and Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta 1793: 1901–1916, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Fetterman JL, Holbrook M, Flint N, Feng B, Breton-Romero R, Linder EA, Berk BD, Duess MA, Farb MG, Gokce N, Shirihai OS, Hamburg NM, and Vita JA. Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis 247: 207–217, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman SA. and Grinstein S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol Rev 262: 193–215, 2014 [DOI] [PubMed] [Google Scholar]
- 23. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FKM, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin K-M, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine J-C, Martin SJ, Martinou J-C, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon H-U, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, and Kroemer G. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25: 486–541, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gump JM, Staskiewicz L, Morgan MJ, Bamberg A, Riches DWH, and Thorburn A. Autophagy variation within a cell population determines cell fate via selective degradation of Fap-1. Nat Cell Biol 16: 47–54, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo F, Li X, Peng J, Tang Y, Yang Q, Liu L, Wang Z, Jiang Z, Xiao M, Ni C, Chen R, Wei D, and Wang G-X. Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng 42: 1978–1988, 2014 [DOI] [PubMed] [Google Scholar]
- 26. Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, Pan Y, and Li XJ. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 8: 812–825, 2012 [DOI] [PubMed] [Google Scholar]
- 27. Harrington EO, Lu Q, and Rounds S. Endothelial cell apoptosis. In: Endothelial Biomedicine, edited by Aird WC. New York: Cambridge University Press, 2007, p. 1081 [Google Scholar]
- 28. Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, and Lee PJ. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arter Thromb Vasc Biol 35: 1166–1178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He C. and Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirota Y, Yamashita S-i, Kurihara Y, Jin X, Aihara M, Saigusa T, Kang D, and Kanki T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy 11: 332–343, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Itoh T, Fujita N, Kanno E, Yamamoto A, Yoshimori T, and Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell 19: 2916–2925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jin M. and Klionsky DJ. Regulation of autophagy: Modulation of the size and number of autophagosomes. FEBS Lett 588: 2457–2463, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang R. and Tang D. Autophagy and ferroptosis—What's the connection? Curr Pathobiol Rep 5: 153–159, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, and Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 163: 737–744, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, and Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato F, Sakao S, Takeuchi T, Suzuki T, Nishimura R, Yasuda T, Tanabe N, and Tatsumi K. Endothelial cell-related autophagic pathways in Sugen/hypoxia-exposed pulmonary arterial hypertensive rats. Am J Physiol Lung Cell Mol Physiol 313: L899–L915, 2017 [DOI] [PubMed] [Google Scholar]
- 37. Kaushik S. and Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol 19: 365–381, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kiffin R, Christian C, Knecht E, and Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell 15: 4829–4840, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim J, Kundu M, Viollet B, and Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of ULK1. Nat Cell Biol 13: 132–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kofler N, Corti F, Rivera-Molina F, Deng Y, Toomre D, and Simons M. The Rab-effector protein RABEP2 regulates endosomal trafficking to mediate vascular endothelial growth factor receptor-2 (VEGFR2)-dependent signaling. J Biol Chem 293: 4805–4817, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kucera A, Bakke O, and Progida C. The multiple roles of Rab9 in the endolysosomal system. Commun Integr Biol 9: e1204498, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, An CH, Shan B, Franks JM, Haley KJ, Owen CA, Tesfaigzi Y, Washko GR, Quackenbush J, Silverman EK, Rahman I, Kim HP, Mahmood A, Biswal SS, Ryter SW, and Choi AM. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest 123: 5212–5230, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lan C, Chen X, Zhang Y, Wang W, Wang WE, Liu Y, Cai Y, Ren H, Zheng S, Zhou L, and Zeng C. Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC Cardiovasc Dis 18: 43, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, and Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee S-J, Smith A, Guo L, Alastalo T-P, Li M, Sawada H, Liu X, Chen Z-H, Ifedigbo E, Jin Y, Feghali-Bostwick C, Ryter SW, Kim HP, Rabinovitch M, and Choi AMK. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med 183: 649–658, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li H, He B, Liu X, Li J, Liu Q, Dong W, Xu Z, Qian G, Zuo H, Hu C, Qian H, Mao C, and Wang G. Regulation on Toll-like receptor 4 and cell barrier function by Rab26 siRNA-loaded DNA nanovector in pulmonary microvascular endothelial cells. Theranostics 7: 2537–2554, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu WC, Chen SB, Liu S, Ling X, Xu QR, Yu BT, and Tang J. Inhibition of mitochondrial autophagy protects donor lungs for lung transplantation against ischaemia-reperfusion injury in rats via the mTOR pathway. J Cell Mol Med 23: 3190–3201, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Y, Shoji-Kawata S, Sumpter RM, Wei Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, Shaw SY, Clarke PGH, Puyal J, and Levine B. Autosis is a Na(+), K(+)-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci U S A 110: 20364–20371, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Longatti A, Lamb CA, Razi M, Yoshimura S-i, Barr FA, and Tooze SA. TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J Cell Biol 197: 659–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu Q, Sakhatskyy P, Grinnell K, Newton J, Ortiz M, Wang Y, Sanchez-Esteban J, Harrington EO, and Rounds S. Cigarette smoke causes lung vascular barrier dysfunction via oxidative stress-mediated inhibition of RhoA and focal adhesion kinase. Am J Physiol Lung Cell Mol Physiol 301: L847–L857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luton F, Klein S, Chauvin JP, Le Bivic A, Bourgoin S, Franco M, and Chardin P. EFA6, exchange factor for Arf6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement. Mol Biol Cell 15: 1134–1145, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maejima Y, Isobe M, and Sadoshima J. Regulation of autophagy by beclin 1 in the heart. J Mol Cell Cardiol 95: 19–25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maniatis NA, Brovkovych V, Allen SE, John TA, Shajahan AN, Tiruppathi C, Vogel SM, Skidgel RA, Malik AB, and Minshall RD. Novel mechanism of endothelial nitric oxide synthase activation mediated by caveolae internalization in endothelial cells. Circ Res 99: 870–877, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Manil-Segalén M, Lefebvre C, Culetto E, and Legouis R. Need an ESCRT for autophagosomal maturation? Commun Integr Biol 5: 566–571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mao M, Yu X, Ge X, Gu R, Li Q, Song S, Zheng X, Shen T, Li X, Fu Y, Li J, and Zhu D. Acetylated cyclophilin A is a major mediator in hypoxia-induced autophagy and pulmonary vascular angiogenesis. J Hypertens 35: 798–809, 2017 [DOI] [PubMed] [Google Scholar]
- 56. Marcos-Ramiro B, García-Weber D, Barroso S, Feito J, Ortega MC, Cernuda-Morollón E, Reglero-Real N, Fernández-Martín L, Durán MC, Alonso MA, Correas I, Cox S, Ridley AJ, and Millán J. RhoB controls endothelial barrier recovery by inhibiting Rac1 trafficking to the cell border. J Cell Biol 213: 385–402, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mari M, Tooze SA, and Reggiori F. The puzzling origin of the autophagosomal membrane. F1000 Biol Rep 3: 25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mathew R, Huang J, and Gewitz MH. Pulmonary artery hypertension: Caveolin-1 and eNOS interrelationship: A new perspective. Cardiol Rev 15: 143–149, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Mizumura K, Cloonan S, Choi ME, Hashimoto S, Nakahira K, Ryter SW, and Choi AMK. Autophagy: Friend or foe in lung disease? Ann Am Thorac Soc 13(Suppl. 1): S40–S47, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mizushima N. and Komatsu M. Autophagy: Renovation of cells and tissues. Cell 147: 728–741, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Moreau K, Renna M, and Rubinsztein DC. Connections between SNAREs and autophagy. Trends Biochem Sci 38: 57–63, 2013 [DOI] [PubMed] [Google Scholar]
- 62. Mu Z, Wang L, Deng W, Wang J, and Wu G. Structural insight into the Ragulator complex which anchors mTORC1 to the lysosomal membrane. Cell Discov 3: 17049, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, and Okada M. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J 28: 477–489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nakahira K, Haspel JA, Rathinam VAK, Lee S-J, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, and Choi AMK. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, and Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461: 654–658, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Orcholski ME, Khurshudyan A, Shamskhou EA, Yuan K, Chen IY, Kodani SD, Morisseau C, Hammock BD, Hong EM, Alexandrova L, Alastalo T-P, Berry G, Zamanian RT, and de Jesus Perez VA. Reduced carboxylesterase 1 is associated with endothelial injury in methamphetamine-induced pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 313: L252–L266, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Piper RC. and Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23: 519–547, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Platta HW. and Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol 23: 393–403, 2011 [DOI] [PubMed] [Google Scholar]
- 69. Pyrzynska B, Pilecka I, and Miaczynska M. Endocytic proteins in the regulation of nuclear signaling, transcription and tumorigenesis. Mol Oncol 3: 321–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rabanal-Ruiz Y, Otten EG, and Korolchuk VI. mTORC1 as the main gateway to autophagy. Essays Biochem 61: 565–584, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rojo de la Vega M, Dodson M, Gross C, Manzour H, Lantz RC, Chapman E, Wang T, Black SM, Garcia JGN, and Zhang DD. Role of Nrf2 and autophagy in acute lung injury. Curr Pharmacol Rep 2: 91–101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rubinsztein DC, Codogno P, and Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11: 709–730, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rusten TE. and Stenmark H. How do ESCRT proteins control autophagy? J Cell Sci 122: 2179–2183, 2009 [DOI] [PubMed] [Google Scholar]
- 74. Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, and Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20: 131–139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, and Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Sakhatskyy P, Gabino Miranda GA, Newton J, Lee CG, Choudhary G, Vang A, Rounds S, and Lu Q. Cigarette smoke-induced lung endothelial apoptosis and emphysema are associated with impairment of FAK and eIF2alpha. Microvasc Res 94: 80–89, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, and Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans 41: 1103–1130, 2013 [DOI] [PubMed] [Google Scholar]
- 79. Sauler M, Bazan IS, and Lee PJ. Cell death in the lung: The apoptosis-necroptosis axis. Annu Rev Physiol 81: 375–402, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schmid SL. Reciprocal regulation of signaling and endocytosis: Implications for the evolving cancer cell. J Cell Biol 216: 2623–2632, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shukla A, Malik M, Cataisson C, Ho Y, Friesen T, Suh KS, and Yuspa SH. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat Cell Biol 11: 777–784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Singh R. and Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab 13: 495–504, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Slavin SA, Leonard A, Grose V, Fazal F, and Rahman A. Autophagy inhibitor 3-methyladenine protects against endothelial cell barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol 314: L388–l396, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Smith EM, Finn SG, Tee AR, Browne GJ, and Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem 280: 18717–18727, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Solano-Gálvez S, Abadi-Chiriti J, Gutiérrez-Velez L, Rodríguez-Puente E, Konstat-Korzenny E, Álvarez-Hernández D-A, Franyuti-Kelly G, Gutiérrez-Kobeh L, and Vázquez-López R. Apoptosis: Activation and inhibition in health and disease. Med Sci 6: pii: , 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stamatovic SM, Johnson AM, Sladojevic N, Keep RF, and Andjelkovic AV. Endocytosis of tight junction proteins and the regulation of degradation and recycling. Ann N Y Acad Sci 1397: 54–65, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stein M, Pilli M, Bernauer S, Habermann BH, Zerial M, and Wade RC. The interaction properties of the human Rab GTPase family—A comparative analysis reveals determinants of molecular binding selectivity. PLoS One 7: e34870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009 [DOI] [PubMed] [Google Scholar]
- 89. Südhof TC. and Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science 323: 474–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sun Q, Westphal W, Wong KN, Tan I, and Zhong Q. Rubicon controls endosome maturation as a Rab7 effector. Proc Natl Acad Sci U S A 107: 19338–19343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sun Y, Li C, Shu Y, Ju X, Zou Z, Wang H, Rao S, Guo F, Liu H, Nan W, Zhao Y, Yan Y, Tang J, Zhao C, Yang P, Liu K, Wang S, Lu H, Li X, Tan L, Gao R, Song J, Gao X, Tian X, Qin Y, Xu K-F, Li D, Jin N, and Jiang C. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci Signal 5: ra16, 2012 [DOI] [PubMed] [Google Scholar]
- 92. Surolia R, Karki S, Kim H, Yu Z, Kulkarni T, Mirov SB, Carter AB, Rowe SM, Matalon S, Thannickal VJ, Agarwal A, and Antony VB. Heme oxygenase-1-mediated autophagy protects against pulmonary endothelial cell death and development of emphysema in cadmium-treated mice. Am J Physiol Lung Cell Mol Physiol 309: L280–L292, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Talaber G, Miklossy G, Oaks Z, Liu Y, Tooze SA, Chudakov DM, Banki K, and Perl A. HRES-1/Rab4 promotes the formation of LC3(+) autophagosomes and the accumulation of mitochondria during autophagy. PLoS One 9: e84392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tekirdag K. and Cuervo AM. Chaperone-mediated autophagy and endosomal microautophagy: Joint by a chaperone. J Biol Chem 293: 5414–5424, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Teng RJ, Du J, Welak S, Guan T, Eis A, Shi Y, and Konduri GG. Cross talk between NADPH oxidase and autophagy in pulmonary artery endothelial cells with intrauterine persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L651–L663, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ulmasov B, Bruno J, Gordon N, Hartnett ME, and Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol 174: 1084–1096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Van Der Sluijs P, Hull M, Zahraoui A, Tavitian A, Goud B, and Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A 88: 6313, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Villasenor R, Kalaidzidis Y, and Zerial M. Signal processing by the endosomal system. Curr Opin Cell Biol 39: 53–60, 2016 [DOI] [PubMed] [Google Scholar]
- 99. Vion AC, Kheloufi M, Hammoutene A, Poisson J, Lasselin J, Devue C, Pic I, Dupont N, Busse J, Stark K, Lafaurie-Janvore J, Barakat AI, Loyer X, Souyri M, Viollet B, Julia P, Tedgui A, Codogno P, Boulanger CM, and Rautou PE. Autophagy is required for endothelial cell alignment and atheroprotection under physiological blood flow. Proc Natl Acad Sci U S A 114: E8675–E8684, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang T, Ming Z, Xiaochun W, and Hong W. Rab7: Role of its protein interaction cascades in endo-lysosomal traffic. Cell Signall 23: 516–521, 2011 [DOI] [PubMed] [Google Scholar]
- 101. Wirth M, Joachim J, and Tooze SA. Autophagosome formation—The role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol 23: 301–309, 2013 [DOI] [PubMed] [Google Scholar]
- 102. Wojciak-Stothard B, Abdul-Salam VB, Lao KH, Tsang H, Irwin DC, Lisk C, Loomis Z, Stenmark KR, Edwards JC, Yuspa SH, Howard LS, Edwards RJ, Rhodes CJ, Gibbs JSR, Wharton J, Zhao L, and Wilkins MR. Aberrant chloride intracellular channel 4 expression contributes to endothelial dysfunction in pulmonary arterial hypertension. Circulation 129: 1770–1780, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yamano K, Fogel AI, Wang C, van der Bliek AM, and Youle RJ. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. Elife 3: e01612, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yan Z, Wang Z-G, Segev N, Hu S, Minshall RD, Dull RO, Zhang M, Malik AB, and Hu G. Rab11a mediates VE-cadherin recycling and controls endothelial barrier function. Arter Thromb Vasc Biol 36: 339–349, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yang J, Yao W, Qian G, Wei Z, Wu G, and Wang G. Rab5-mediated VE-cadherin internalization regulates the barrier function of the lung microvascular endothelium. Cell Mol Life Sci 72: 4849–4866, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yao P, Zhao H, Mo W, and He P. Laminar shear stress promotes vascular endothelial cell autophagy through upregulation with Rab4. DNA Cell Biol 35: 118–123, 2016 [DOI] [PubMed] [Google Scholar]
- 107. Zada S, Noh HS, Baek SM, Ha JH, Hahm JR, and Kim DR. Depletion of p18/LAMTOR1 promotes cell survival via activation of p27kip1-dependent autophagy under starvation. Cell Biol Int 39: 1242–1250, 2015 [DOI] [PubMed] [Google Scholar]
- 108. Zerial M. and McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107, 2001 [DOI] [PubMed] [Google Scholar]
- 109. Zhang CF, Zhao FY, Xu SL, Liu J, Xing XQ, and Yang J. Autophagy in pulmonary hypertension: Emerging roles and therapeutic implications. J Cell Physiol 234: 16755–16767, 2019 [DOI] [PubMed] [Google Scholar]
- 110. Zhang D, Zhou J, Ye LC, Li J, Wu Z, Li Y, and Li C. Autophagy maintains the integrity of endothelial barrier in LPS-induced lung injury. J Cell Physiol 233: 688–698, 2018 [DOI] [PubMed] [Google Scholar]
- 111. Zhang J, Huang J, Qi T, Huang Y, Lu Y, Zhan T, Gong H, Zhu Z, Shi Y, Zhou J, Yu L, Zhang X, Cheng H, and Ke Y. SHP2 protects endothelial cell barrier through suppressing VE-cadherin internalization regulated by MET-Arf1. FASEB J 33: 1124–1137, 2019 [DOI] [PubMed] [Google Scholar]
- 112. Zhang J, Wang J, Zheng Z, Tang J, Fan K, Guo H, and Wang J. Participation of autophagy in lung ischemia-reperfusion injury in vivo. J Surg Res 182: e79–e87, 2013 [DOI] [PubMed] [Google Scholar]
- 113. Zhang Y, Jiang G, Sauler M, and Lee PJ. Lung endothelial HO-1 targeting in vivo using lentiviral miRNA regulates apoptosis and autophagy during oxidant injury. FASEB J 27: 4041–4058, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhen Y. and Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 128: 3171, 2015 [DOI] [PubMed] [Google Scholar]
- 115. Zhou F, Wu Z, Zhao M, Murtazina R, Cai J, Zhang A, Li R, Sun D, Li W, Zhao L, Li Q, Zhu J, Cong X, Zhou Y, Xie Z, Gyurkovska V, Li L, Huang X, Xue Y, Chen L, Xu H, Xu H, Liang Y, and Segev N. Rab5-dependent autophagosome closure by ESCRT. J Cell Biol 18: 1908–1927, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]