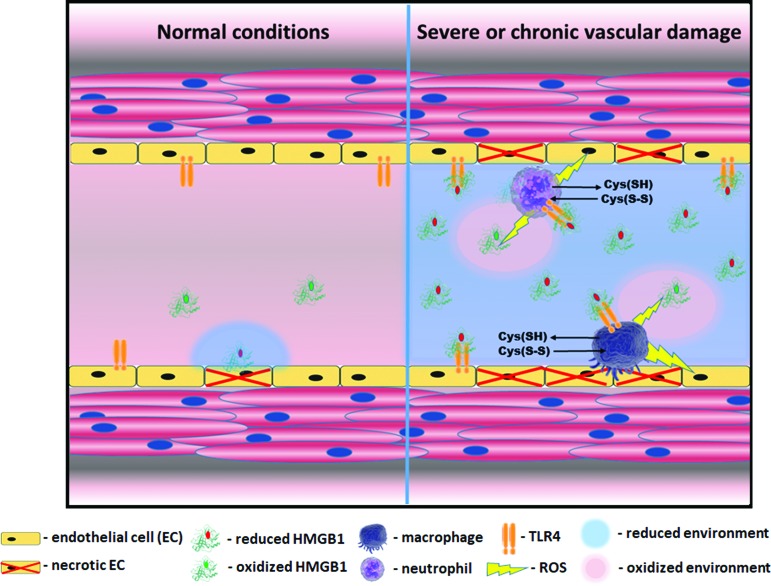
FIG. 9.
Necrosis mediated activation of vascular inflammation. HMGB1 is active in its reduced state, whereas oxidation inhibits its activity. Therefore, the duration of HMGB1 signaling depends on the redox status of the extracellular environment, where it gets released on cell death. In a healthy state (left part of a scheme), even in a case of occasional necrosis, oxidized state of the extracellular environment ensures a quick inhibition of HMGB1. However, if the level of necrosis is relatively high like it was found in males, the local extracellular environment shifts to become more reduced due to the release of high amounts of intracellular glutathione and other thiols (right part of a scheme). This reduced environment prolongs HMGB1-mediated signaling. The reduced HMGB1 binds to a TLR4 receptor of endothelial cells and induces their activation. Active endothelial cells release cytokines, which attract macrophages and leukocytes to the site of damage and express the adhesive molecules that promote the recruitment of inflammatory cells. Activated macrophages and neutrophils, by producing ROS, oxidize HMGB1, but at the same time may additionally injure endothelial cells and, thus, promote damage-mediated inflammation. Moreover, activation of pattern recognition receptors such as TLR4 and RAGE induces further propagation of reductive stress by activation of cystine/cysteine redox cycle and secretion of large amounts of reduced free cysteine outside the cell (45). RAGE, receptor for advanced glycation end products; ROS, reactive oxygen species. Color images are available online.