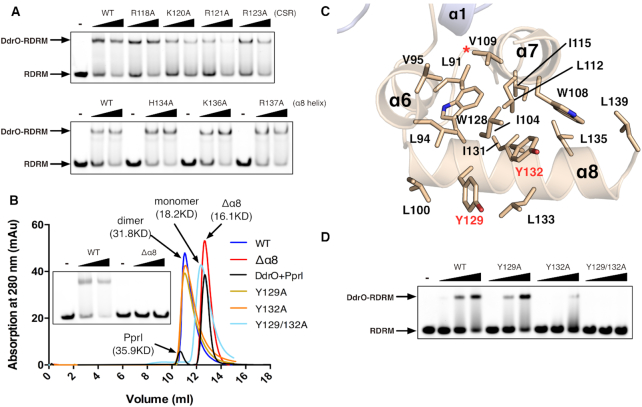
Figure 4.
The derepression mechanism of DG-DdrO. (A) EMSA showing the unaffected promoter DNA binding of DG-DdrO mutant proteins. 5′-FAM-labeled DNA containing RDRM sequence (100 nM) was incubated with 1 or 2 μM of DdrO mutant protein. (B) Size exclusion chromatography of wild type, mutant (Y129A, Y132A and Y129/132A), truncated (Δα8) DG-DdrO proteins and cleaved DG-DdrO protein (DdrO+PprI, cleavage reaction at 45°C for 30 min) on Superdex 75 10/300 GL column. The peaks correspond to monomeric or dimeric DG-DdrO proteins and their calculated molecular weights are labeled and colored differently. (C) A cut away view shows that the C-terminal domain of DG-DdrO forms a stable hydrophobic core. Conserved hydrophobic residues are shown as stick and labeled. The red star indicates the cleavage site. (D) EMSA assays of mutant DG-DdrO proteins using the same reaction conditions as in panel A.