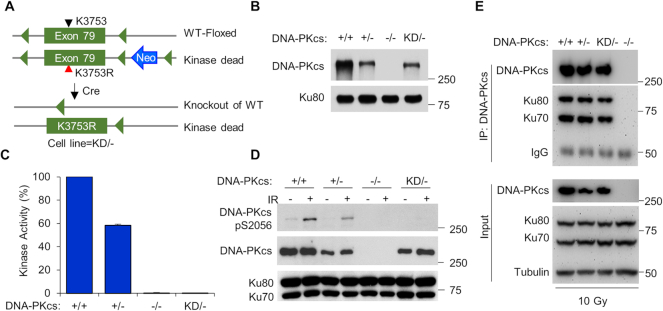
Figure 1.
Generation and initial characterization of the DNA-PKcs kinase-dead (KD) HCT116 cell line. (A) Basic schematic of the method used to generate the HCT116 DNA-PKcs kinase dead (KD/−) cell line. (B) Expression level of DNA-PKcs in HCT116 DNA-PKcs +/+, +/−, −/−, and KD/− cells as assessed by western blotting. (C) Measurement of DNA-PKcsin vitro kinase activity. Nuclear extracts from the HCT116 DNA-PKcs +/+, +/−, −/−, and KD/− cells were examined for their ability to phosphorylate a biotin-tagged H2AX peptide. H2AX phosphorylation was observed in the −/− cell line and this was subtracted from the other samples’ readouts. The 100% kinase activity was normalized using the +/+ cell lysate results. The data are presented as the mean ± SD from three individual experiments. (D) Measurement of DNA-PKcsin vivo kinase activity. The HCT116 DNA-PKcs +/+, +/−, −/−, and KD/− cell lines were mock-treated or γ-irradiated with a dose of 10 Gy and allowed to recover for 30 min. Cell extracts were prepared and western blot analysis was performed to assess autophosphorylation of DNA-PKcs at serine 2056. Immunoblotting of Ku70 and Ku80 were used as loading controls. (E) The interaction between DNA-PKcs and the Ku70/80 heterodimer is not affected by inactivating the kinase activity of DNA-PKcs. DNA-PKcs was immunoprecipitated from the HCT116 +/+, +/−, −/−, and KD/− cell lines 5 min after being irradiated with 10 Gy of γ-rays. The immunoprecipitates were analyzed by western blotting with anti-DNA-PKcs, Ku80, and Ku70 antibodies. Tubulin was used as a loading control for the input of each immunoprecipitation assay.