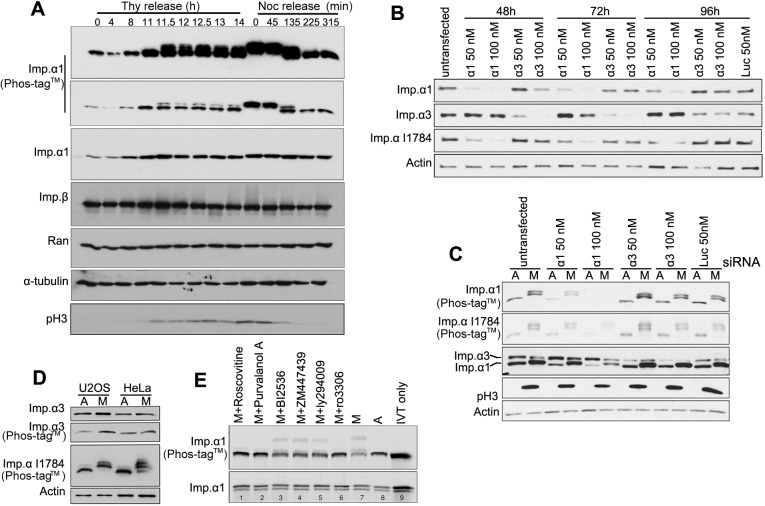
Fig. 2.
Phosphorylation of importin-α1 during the cell cycle. (A) HeLa cells were arrested at G1/S via a double-thymidine blockade, followed by release at different time points, or were arrested at mitosis via a double-thymidine blockade, followed by release into nocodazole blockade and release at different time points. Cells were collected and subjected to Phos-tag™ SDS-PAGE and standard SDS-PAGE and immunoblotting. Histone H3 phosphorylated at S10 (pH3) was detected to indicate mitosis. α-Tubulin serves as a loading control. (B) Importin-α1 is the most abundant isoform recognized by anti-importin-α antibody I1784. HeLa cells were transfected with importin-α1, importin α3 and luciferase siRNA at the indicated concentration for 48–96 h. Western blotting using the mentioned antibodies revealed the knockdown efficiency. (C) Importin-α1 is the main isoform phosphorylated in mitosis. HeLa cells were transfected with importin-α1, importin α3 and luciferase siRNA at the indicated concentration for 96 h. Asynchronous and mitotic cell lysates were subjected to standard and Phos-tag™ SDS-PAGE, followed by western blotting using the indicated antibodies. (D) Importin-α3 is not phosphorylated in mitosis. Asynchronous and mitotic cell lysates of U2OS and HeLa cells were subjected to standard and Phos-tag™ SDS-PAGE, followed by western blotting using anti-importin-α3 (Abcam, ab6039) and anti-importin-α I1784 antibodies. Actin served as a loading control. (E) IVT importin-α1 phosphorylation is blocked by CDK1 inhibitors. IVT-imp-α1 was incubated in mitotic cell extracts with the addition of a range of kinase inhibitors for 30 min at 30°C. Reactions were stopped by the addition of 2× SDS sample buffer, boiled for 3 min and spun for 5 min. The resultant supernatants were subjected to standard and Phos-tag™ SDS-PAGE. Autoradiography of Phos-tag™ gels showed that the CDK1 inhibitors roscovitine, purvalanol A and Ro3306 blocked IVT-imp-α1 phosphorylation. A, asynchronous; M, mitotic; IVT only, recombinant protein alone without treatment.