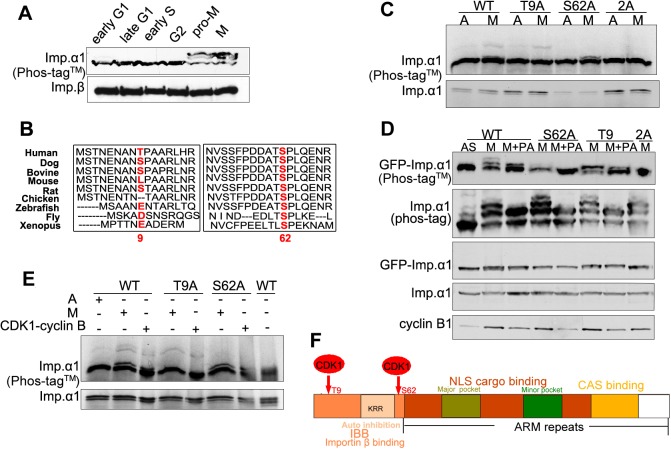
Fig. 3.
Importin-α1 is phosphorylated at T9 and S62 by CDK1–cyclin B1 in mitosis. (A) Importin-α1 is phosphorylated in mitosis at at least two sites. Early S and G2 phase cell extracts were prepared from synchronous HeLa cells at 1 h and 8 h after release from double-thymidine blockade; M phase, early G1 and late G1 cell extracts were prepared from synchronized HeLa cells at 0 h, 4 h and 9 h after release from nocodazole arrest. Cell extracts were subjected to Phos-tag™ SDS-PAGE and were analyzed by immunoblotting with antibodies as indicated. (B) Sequence alignment of importin-α1 among different species. Alignment of the N-terminal region of importin-α1 among different species performed using the CLUSTAL OMEGA multiple alignment tool on the EMBL-EBI website (http://www.ebi.ac.uk/Tools/msa/clustalo/). T9 and S62 are highlighted in red. UniProt identifiers: IMA1_HUMAN, E2R6L9_CANFA, Q3SYV6_BOVIN, IMA1_MOUSE, Q9Z0N9_RAT, F1NJS6_CHICK, Q6DI01_DANR, IMA_DROME, IMA1_XENLA. (C) HeLa cells were transiently transfected with GFP–importin-α1 WT, GFP–importin-α1 S62A, GFP–importin-α1 T9A or GFP-importin-α1 2A. After 17 h of treatment with 100 ng/ml of nocodazole, cells were treated with the CDK inhibitor purvalanol A (+PA) for 20 min. Asynchronous (labeled A or AS) and nocodazole-arrested mitotic cell lysates (labeled M) were collected as controls. Cell extracts were subjected to Phos-tag™ and standard SDS-PAGE and immunoblotting with the indicated antibodies. (D) T9 and S62 double mutation abolished the mitotic phosphorylation of importin-α1. IVT importin-α1 WT, alanine and double-alanine mutants were incubated in mitotic cell extracts for 30 min at 30°C. The reactions were stopped by the addition of 2× SDS sample buffer, boiled for 3 min and centrifuged for 5 min. The resultant supernatants were subjected to standard and Phos-tag™ SDS-PAGE. Autoradiograph of the Phos-tag™ gel showed that double-alanine mutation of T9 and S62 abolished the mitotic phosphorylation of IVT importin-α1. (E) Phosphorylation of IVT importin-α1 by purified CDK1–cyclin B1. IVT importin-α1 WT, T9A and S62A were incubated with asynchronous, mitotic cell extracts or purified recombinant CDK1–cyclin B1 protein for 30 min at 30°C. Reactions were stopped by the addition of 2× SDS sample buffer, boiled for 3 min and centrifuged for 5 min. The resultant supernatants were subjected to standard and Phos-tag™ SDS-PAGE. Autoradiography of a Phos-tag™ gel showed that CDK1–cyclin B1 could phosphorylate IVT importin-α1 in the same manner as the cell extracts. Point mutations at T9 and S62 also blocked the phosphorylation by CDK1–cyclin B1. CDK1–cyclin B1 storage buffer was used as the control to eliminate a possible non-specific effect on phosphorylation. (F) A schematic diagram showing the functional domains and CDK1 phosphorylation sites of importin-α1.