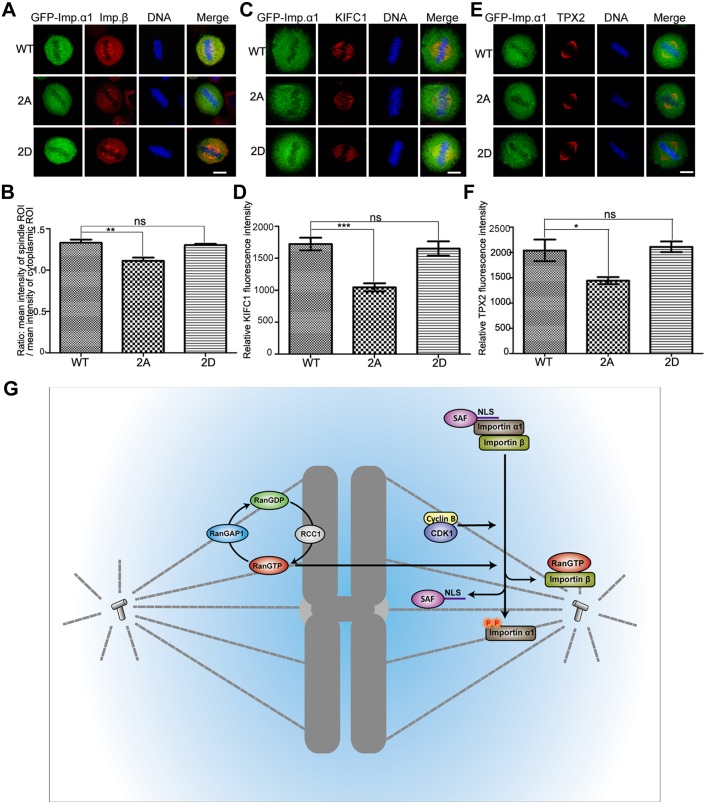
Fig. 8.
Phosphorylation of importin-α1 is necessary for the localization of importin-β and SAFs on the mitotic spindle. (A–F) HeLa cells were transfected with GFP–importin-α1 WT, GFP–importin-α1 2A and GFP–importin-α1 2D for 48 h and then were fixed with 3.7% PFA in PEM buffer and immunostained with anti-importin-β antibody (A) or were fixed with methanol and immunostained with (C) anti-KIFC1 or (E) anti-TPX2 antibody. DNA (blue) was stained using DAPI. Typical images are shown. Scale bars: 10 µm. (B) The ratio between the intensity of importin-β in spindle ROI and cytoplasm ROI was measured and statistically calculated. Experiments were repeated three times, and at least 100 cells were measured for each group. Results are mean±s.d. The intensity of spindle-located (D) KIFC1 and (F) TPX2 was measured by Volocity software (Perkin Elmer) and statistics calculated by GraphPad Prism5 software. *P<0.05, **P<0.01, ***P<0.001; ns, not significant (Student's t-test). (G) A model for the dual control of importin-regulated mitotic spindle assembly by Ran-GTP and CDK1–cyclinB1. The chromatin-localized guanine-nucleotide exchange factor RCC1 generates RanGTP at chromosomes, providing a spatial signal for spindle assembly. In addition, phosphorylation of importin-α1 by CDK1–cyclin B1 attenuates the interaction of importin-α1 with importin-β, promoting the release of NLS-containing spindle assembly factors (SAFs) from importin-α1.