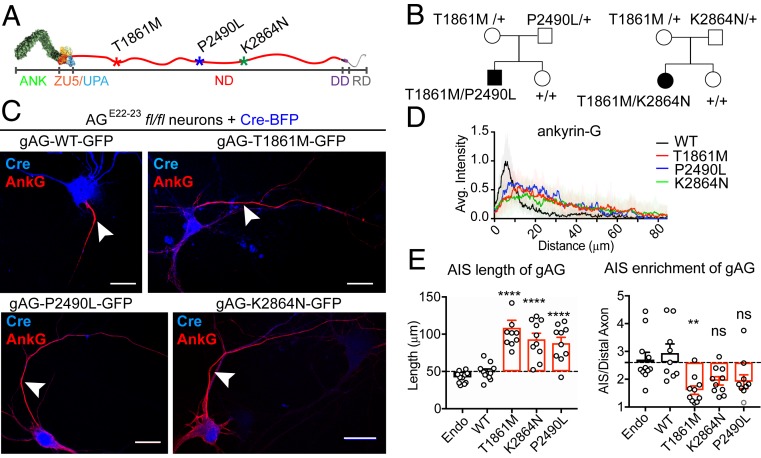
Fig. 1.
Newly identified human neurodevelopment disorder mutations of ANK3 impair gAnkG targeting to the AIS. (A) Schematic of the gAnkG polypeptide. Ankyrin repeats (ANK), ZU5/UPA domain, neurospecific domain (ND), death domain (DD), and regulatory domain (RD) are indicated. Asterisks indicate amino acid positions of human mutations. (B) Pedigrees of affected individuals with corresponding compound heterozygous mutations. (C) The 3 DIV hippocampal neurons from AGE22−23fl/fl mice were cultured and cotransfected with Cre-2A-BFP and plasmids encoding gAnkG-EGFP that were either WT or bearing human mutations. Transfected neurons were fixed at 7 DIV and stained with an AnkG antibody. Representative images are shown for Cre (blue) and AnkG staining (red) (Scale bar, 20 μm.) (D) Average intensity of AnkG staining at the AIS is plotted and aligned for neurons transfected with WT gAnkG (black) or gAnkG with human mutations (red for T1861M, blue for P2490L, and green for K2864N). n = 10 and results are repeated in 3 independent neuronal cultures. (E) Quantification of the length and the enrichment of AnkG at the AIS of transfected neurons. Mean ± SEM; **P = 0.005, ****P < 0.0001; 1-way ANOVA followed by Dunnett’s multiple comparisons test; n = 10; experiments were repeated in 3 independent cultures; ns, not significantly different.