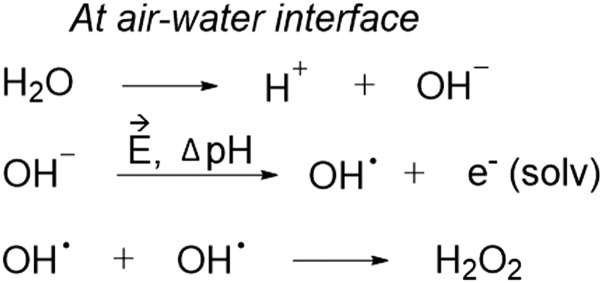
Fig. 5.
Proposed mechanism to form H2O2 at the air−water interface of microdroplets. First, the autoionization of water into H+ and OH− readily occurs at and near the air−water interface of the microdroplet. Then, due to the pH gradient and electric field, OH radicals are formed, releasing a solvated electron. Finally, 2 OH radicals at and near the water microdroplet interface recombine to form H2O2.