Figure. 2. Overview of NAFLD pathogenesis and sex differences.
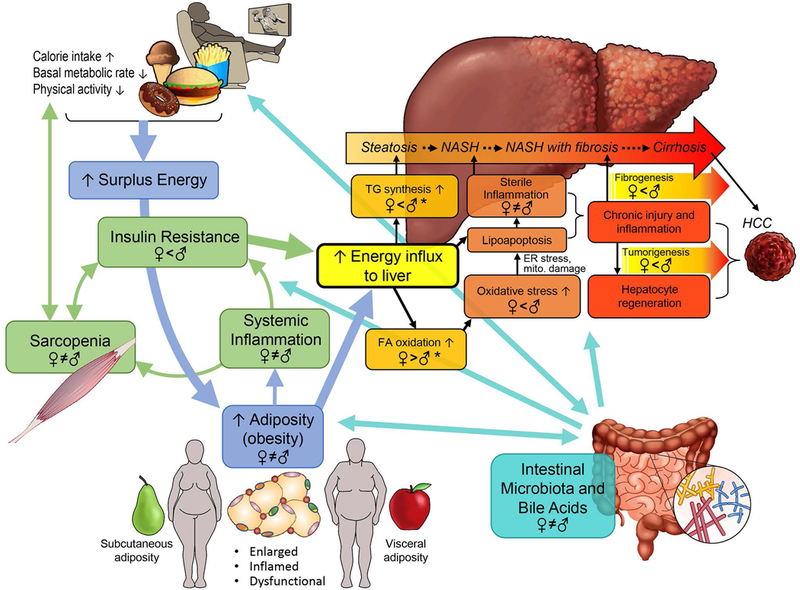
TG: triglycerides. Women and men store surplus calories differently: gluteo-femoral subcutaneous in women vs. visceral adiposity in men. Enlarged, dysfunctional adipose tissue, especially visceral adiposity, leads to systemic inflammation and insulin resistance, which facilitates energy influx to the liver and increases metabolic stress in hepatocytes. Sarcopenia exacerbates these changes, by generating a vicious cycle. When hepatocytes fail to adapt, the increased metabolic stress triggers oxidative stress or direct toxic effect of free FA on hepatocytes, and induces lipoapoptosis, which, in turn, leads to sterile inflammation. Chronic inflammation promotes fibrosis, cirrhosis, and tumorigenesis. Intestinal microbiota and BA play pivotal roles in regulating NAFLD pathogenesis in a multi-phasic manner while interacting with key players. Known sex differences and hormonal effects are depicted in a mechanism-specific way for further discussion in this review. Sex differences in TG synthesis, FA oxidation, and oxidative stress are not covered in this review. Sex difference in oxidative stress is well accepted. * see reference (85)
