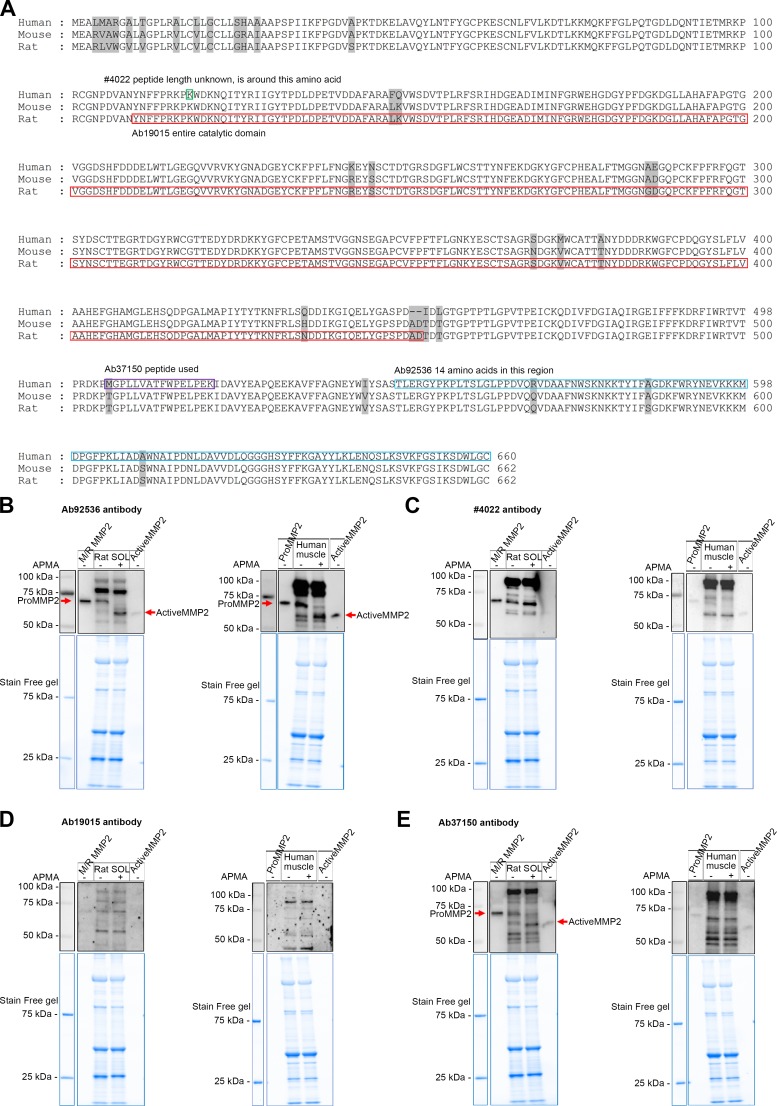
Fig. 1.
Testing the appropriateness of matrix metalloproteinase 2 (MMP2) antibodies in Western blotting. A: sequence alignment for immunogens of the MMP2 antibodies using basic local alignment search tool (BLAST) similarity analysis of MMP2 in human, mouse, and rat species. The antigen of the antibody no. 4022 is around amino acid 116 (green rectangle), the catalytic domain of rat MMP2 is in the red rectangle, and the antibody Ab19015 uses an antigen of this full region; the antibody Ab37150 peptide is in the purple rectangle and the antibody Ab92536 peptide is in the blue rectangle. The number of amino acids is shown at right. Nonconserved amino acids of MMP2 from 3 species are highlighted in gray. B–E: p-aminophenylmercuric acetate (APMA)-treated (37°C for 1 h) or untreated rat soleus (SOL) and human vastus lateralis muscle homogenates (100 μg muscle wet wt), along with mouse/rat pure pro-MMP2 protein (M/R MMP2) and human pro- and active MMP2 pure proteins (0.2 ng) probed for MMP2 with Ab92536 (B), no. 4022 (C), Ab19015 (D), and Ab37150 (E); molecular mass markers are shown at left. Stain-Free (blue) gels indicate total protein loaded. B: specific bands of pro- and active MMP2 (red arrows) were detected with Ab92536 in APMA-treated and untreated rat soleus and human vastus lateralis muscle homogenates. C and D: no. 4022 and Ab19015 failed to detect pro- and active MMP2 in rat soleus and human vastus lateralis muscle homogenates. E: Ab37150 detected pro- and active MMP2 (red arrows) in APMA-treated and untreated rat soleus muscle homogenate; many nonspecific bands were detected, suggesting that Ab37150 may not be suitable for use in immunofluorescence studies; Ab37150 failed to detect pro- and active MMP2 in human vastus lateralis muscle. Aliquots of the same muscle samples were used in B–E. In addition to specfic bands (B and E), all antibodies detected nonspecific bands.