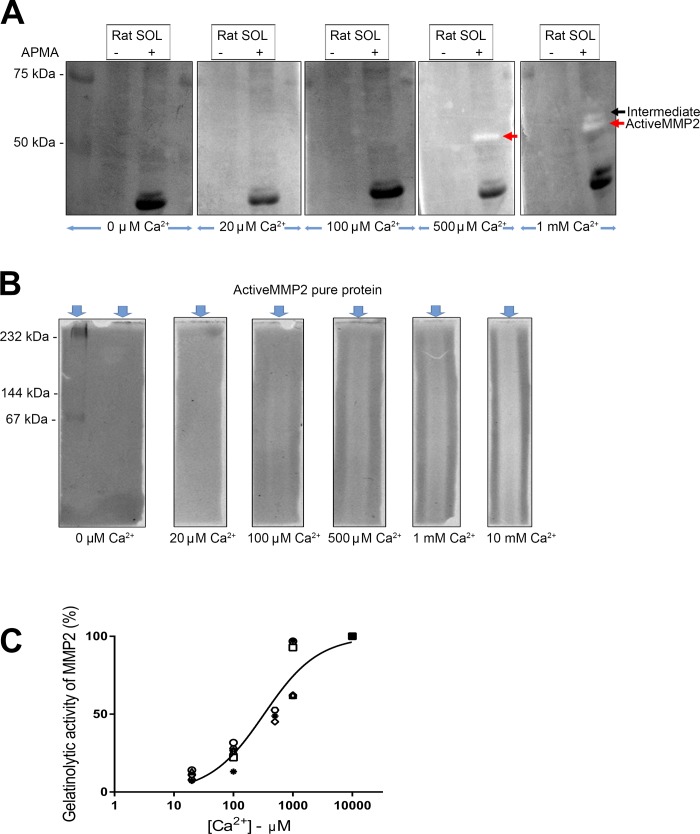
Fig. 7.
Influence of Ca2+ on gelatinolytic activity of matrix metalloproteinase 2 (MMP2). A: representative zymography for detection of gelatinolytic activity of MMP2 in rat soleus (SOL) muscle, with or without p-aminophenylmercuric acetate (APMA) treatment, incubated in different free [Ca2+] from 0 µM to 1 mM, and 50 mM Tris·HCl, 150 mM NaCl, 1 µM ZnSO4, and 0.002% NaN3 (pH 7.5). White bands indicate gelatinolytic activity of pro- and active MMP2 in muscle. A similar pattern of MMP2 activity was observed in 4 independent experiments. B: gelatinolytic activity of active MMP2 pure protein (25 ng) under native conditions, with incubation in 0 µM to 10 mM free Ca2+ and other constituents described as in A. White regions indicate gelatinolytic activity of active MMP2. For A and B, zymography gels incubated in different Ca2+ concentrations were imaged together. C: relative gelatinolytic activity of active MMP2 detected by zymography under native conditions with different calcium concentrations ([Ca2+]). Values from 6 independent experiments are shown as different symbols. The y-axis shows activity relative to that observed with 10 mM Ca2+ (all values expressed relative to 10 mM Ca2+), and x-axis shows free [Ca2+] on log10 scale. Hill curve was fitted to data.