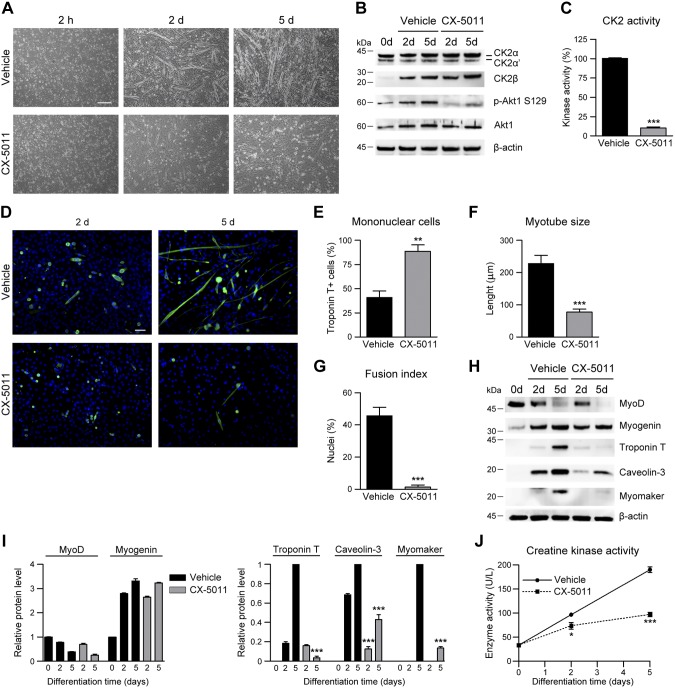
Figure 4.
Inhibition of CK2 activity impairs myoblast differentiation in primary muscle precursor cells. Muscle precursor cells derived from newborn C57BL/6 mice differentiated in the presence of vehicle or 2 μM CX-5011 were analyzed at the indicated times. A) Cell morphology assessed by bright-field microscopy (n = 3). Scale bar, 200 μm. B) Representative immunoblotting (n = 3). C) In vitro CK2 kinase activity tested in cellular lysates toward eIF2β (1–22) peptide substrate and expressed as percentage relative to controls (n = 3; means ± sem). D) Immunofluorescence of troponin T (green) in muscle precursor cells after 2 and 5 d in differentiating medium in the presence of vehicle or 2 μM CX-5011. Hoechst (blue) stains nuclei (n = 3). Scale bar, 50 μm. E) Quantification of mononucleated troponin T–positive cells at 5 d of differentiation and treated as in D. Data are expressed as percentages relative to the total troponin T–positive cells (n = 3; means ± sem). F) Quantification of troponin T–positive primary myotube length at 5 d of differentiation from images obtained as in D (n = 3; means ± sem). G) Fusion index measured in images obtained as in D, defined as the percentage of nuclei present in troponin T–positive primary myotubes at 5 d of differentiation of which cells containing ≥3 nuclei are considered myotubes (n = 3; means ± sem). H–J) Differentiating primary muscle cells were treated with 2 μM CX-5011 and compared with vehicle-treated cells at each indicated time point. H) Representative immunoblotting (n = 3). I) Densitometric quantification of the immunostained bands obtained as in H. Values were normalized to β-actin amount and relative to undifferentiated cells (for MyoD and myogenin) or to 5-d differentiated cells (for troponin T, caveolin-3, and myomaker. n = 3; means ± sem. J) Creatine kinase activity (n = 3; means ± sem). p-Akt, phosphorylated Akt. *P < 0.05, **P < 0.01, ***P < 0.0001 vs. vehicle treated primary muscle cells.