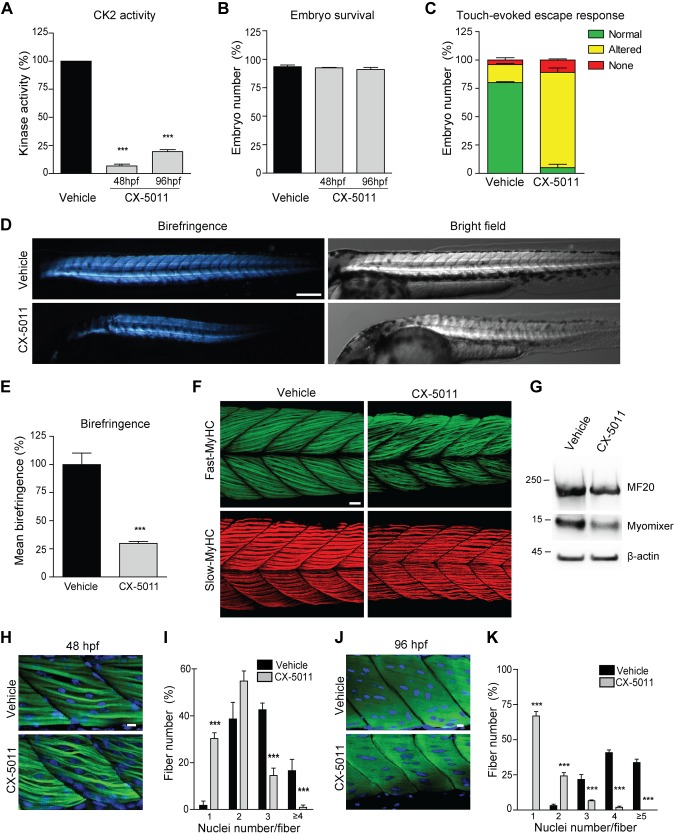
Figure 6.
Inhibition of CK2 activity impairs in vivo skeletal muscle development in zebrafish. Developing zebrafish were treated with vehicle (fish water) or 1 μM CX-5011 at 20 hpf and analyzed at 48 and 96 hpf. A) In vitro CK2 activity tested toward CK2tide substrate in fish extracts and expressed as percentage relative to vehicle-treated samples (n = 30 fish/group; means ± sem). B) Zebrafish viability assessed as the percentage of living animals and expressed as percentage relative to the initial number of fertilized eggs (n = 200 vehicle-treated and n = 250 CX-5011–treated animals from 3 independent experiments). C) Zebrafish locomotor activity assessed by touch-evoked escape response test at 48 hpf. The embryo response to a gentle touch of the tail was classified as follows: “normal” when embryos swim away (green), “altered” when embryos move to a lesser extent or in abnormal way (yellow), or “none” when embryos do not move (red). Results are expressed as percentage of embryos showing the indicated class of response (n = 100 vehicle-treated and n = 80 CX-5011–treated embryos from 3 independent experiments; means ± sem). D) Representative birefringence (left panels) and bright-field (right panels) images of zebrafish embryos at 48 hpf. Scale bar, 250 μm. E) Quantification of muscle birefringence in zebrafish embryos obtained as in D. Data are expressed as percentage of vehicle-treated zebrafish mean birefringence value (n = 30 vehicle-treated and n = 40 CX-5011–treated embryos stained as in H; means ± sem). F) Maximum intensity confocal projections of zebrafish midtrunk somites (lateral view, anterior to the left) at 48 hpf, immunostained for fast (F310 antibody, green) or slow (F59 antibody, red) fiber type–specific myosin heavy chain (MyHC). Scale bar, 20 μm. G) Representative immunoblotting analysis of zebrafish embryo extracts at 48 hpf (n = 30 embryos/group from 3 independent experiments). H) Confocal images of 48-hpf zebrafish midtrunk somites (lateral view, anterior to the left) immunostained for fast fiber type–specific myosin (F310 antibody; green) and counterstained with Hoechst (blue) to detect nuclei. Scale bar, 10 μm. I) Number of nuclei per muscle fiber in 48 hpf zebrafish midtrunk somites stained as in H. Data are presented as percentage of fibers with the indicated number of nuclei in CX-5011- (n = 294 fibers) vs. vehicle-treated embryos (n = 230 fibers). Means ± sem. J) Confocal images of 96-hpf zebrafish midtrunk somites (lateral view, anterior to the left) immunostained for fast fiber type–specific myosin (F310 antibody; green) and counterstained with Hoechst (blue) to detect nuclei. Scale bar, 10 μm. K) Number of nuclei per muscle fiber in 96-hpf zebrafish midtrunk somites stained as in J. Data are presented as percentage of fibers with the indicated number of nuclei in CX-5011- (n = 357 fibers) vs. vehicle-treated embryos (n = 290 fibers). Means ± sem. ***P < 0.0001.