Abstract
Recent reports suggest that the Hippo signaling pathway influences ovarian follicle development; however, its exact roles remain unknown. Here, we examined the ovarian functions of the Hippo kinases large tumor suppressors (LATS)1 and 2, which serve to inactivate the transcriptional coactivators Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). Inactivation of Lats1/2 in murine granulosa cells either in vitro or in vivo resulted in a loss of granulosa cell morphology, function, and gene expression. Mutant cells further underwent changes in structure and gene expression suggestive of epithelial-to-mesenchymal transition and transdifferentiation into multiple lineages. In vivo, granulosa cell–specific loss of Lats1/2 caused the ovarian parenchyma to be mostly replaced by bone tissue and seminiferous tubule-like structures. Transdifferentiation into Sertoli-like cells and osteoblasts was attributed in part to the increased recruitment of YAP and TAZ to the promoters of sex-determining region Y box 9 and bone γ-carboxyglutamate protein, key mediators of male sex determination and osteogenesis, respectively. Together, these results demonstrate for the first time a critical role for Lats1/2 in the maintenance of the granulosa cell genetic program and further highlight the remarkable plasticity of granulosa cells.—Tsoi, M., Morin, M., Rico, C., Johnson, R. L., Paquet, M., Gévry, N., Boerboom, D. Lats1 and Lats2 are required for ovarian granulosa cell fate maintenance.
Keywords: transdifferentiation, Sox9, Bglap, RUNX2, Hippo
Hippo is an evolutionarily conserved signaling pathway with a well-established role in organ size determination (1). It also acts to regulate processes such as cell fate determination, differentiation, proliferation, and apoptosis in a variety of cell types during embryogenesis (2, 3). Hippo has no known specific ligands or receptors, but rather is activated by intracellular and extracellular cues, including the establishment of cell-cell contacts and cytoskeletal changes (4, 5). The pathway consists of a core kinase cascade beginning with the mammalian STE20-like protein kinases (MSTs)1 and 2. MST1 and MST2 function in a redundant manner to phosphorylate the scaffold protein Salvador and Mps One Binder kinase activators 1A and 1B (6, 7). Whereas the interaction between phosphorylated (phospho-)Salvador and MST1/2 serves to enhance kinase activity, phospho–Mps One Binder kinase activators 1A and 1B bind an autoinhibitory motif within the kinases large tumor suppressors (LATS)1 and 2, which permit their subsequent phosphorylation (and activation) by MST1/2 (8, 9). LATS1 and 2 both function to phosphorylate the transcriptional coregulators Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ, also known as the WW domain containing transcription regulator 1). Upon phosphorylation, YAP and TAZ are sequestered in the cytoplasm and degraded by the cellular proteasomal machinery (5, 10–13). Disruption of the Hippo kinase cascade allows YAP and TAZ proteins to escape phosphorylation, accumulate within the cell, and translocate to the nucleus, where they can bind to several transcription factors, notably those of the TEA domain transcription factor (TEAD) and runt-related transcription factor (RUNX) families (14–16). This results in the modulation of the transcriptional activity of a variety of target genes in a cell type- and context-specific manner (2, 3).
The Hippo pathway is required to direct cell fate specification starting at very early stages of preimplantation embryonic development. Notably, a series of studies have shown that Hippo signaling must be differentially regulated in the inner and outer cells of the morula to permit their adoption of the inner cell mass or trophectoderm cell fates (17). YAP localizes to the nucleus in the outer cells, which coactivates TEAD4 and the transcription of trophectoderm-specific genes, whereas YAP localizes to the cytoplasm in the inner cells (18). Inactivation of Tead4 leads to all cells adopting the inner cell mass fate (19). Hippo subsequently acts in different progenitor cell types to direct fate specification in a variety of embryonic and adult tissues (20). For instance, in the liver, the differentiation of hepatoblasts and progenitor cells into either hepatocytes or cholangiocytes is determined by YAP and TAZ expression and activity (21, 22). Likewise, YAP and TAZ activity is necessary and sufficient for the pluripotent progenitor cells of the optic vesicle to adopt the retinal pigment epithelial cell fate (23). In the kidney, Hippo signaling also appears to direct progenitor cells to give rise to either nephron epithelial cells or myofibroblasts (24). The ability of the Hippo pathway to direct cell fate decisions is further illustrated by its ability to induce transdifferentiation in cells already committed to a particular fate. For instance, YAP overexpression in adult hepatocytes causes them to transdifferentiate into biliary epithelial cells (25). Likewise, Hippo signaling can alter cancer cell fate decisions, such as the transdifferentiation of lung adenocarcinoma to squamous cell carcinoma (26).
With most research to date having focused on Hippo’s involvement in embryogenesis and cancer, Hippo signaling in postdevelopmental, physiologic contexts has only recently become intensively studied. In the ovary, early evidence of a role for Hippo signaling in follicle development came with the phenotypic analysis of Lats1 knockout (KO) mice. The latter were found to be subfertile and their ovaries contained reduced numbers of antral follicles, no corpora lutea, and developed stromal tumors later in life (27). More recently, Kawamura et al. studied follicle growth that is induced by ovarian injury, such as that which occurs when ovarian wedge resection, drilling, or grafting procedures are used in the context of infertility treatments. Using a mouse ovary fragmentation and allotransplantation model, they showed that follicle growth is associated with the disruption of Hippo-pathway signaling, as evidenced by a decrease in YAP phosphorylation and an increase in the mRNA levels of YAP-TEAD transcriptional targets (28). The latter study also showed that fragmentation-induced follicle growth could be blocked with verteporfin, a small molecule inhibitor of the interaction between YAP and TEAD (29). In a follow-up study, the same group showed that drugs that promote actin polymerization could enhance follicle growth and do so by increasing nuclear accumulation of YAP and mRNA levels of its transcriptional targets (30). Together these studies suggest that Hippo signaling is a negative regulator of follicle growth, at least in the context of ovarian injury.
Based on the aforementioned studies, we sought to determine if Hippo signaling plays a role in the context of physiologic, gonadotropin-driven ovarian follicle development. Using a conditional gene-targeting approach to inactivate Lats1 and 2 in ovarian granulosa cells, we unexpectedly found that loss of Hippo signaling causes rapid loss of granulosa cell fate. The targeted cells seemed to undergo epithelial-to-mesenchymal transition (EMT) with apparent transdifferentiation into multiple cell types, notably leading to the formation of seminiferous tubule-like structures and bone. Aberrant YAP- and TAZ-mediated transcriptional activation of genes that drive male sex determination and osteogenesis was shown to be, at least in part, the mechanism underlying the transdifferentiation phenotype. Together, our findings indicated a previously unsuspected role for Hippo signaling in maintaining granulosa cell fate.
MATERIALS AND METHODS
Animal model
Mice bearing floxed alleles for Lats1, Lats2, and Lats1/2 (Lats1tm1.1Jfm and Lats2tm1.1Jfm, hereafter Lats1flox/flox, Lats2flox/flox, and Lats1flox/flox;Lats2flox/flox) were generated as previously described in Heallen et al. (31). These mice were mated to the Tg[cytochrome P450 (CYP)19A1-cre]1Jri (hereafter CYP19-cre) strain [courtesy of Jan Gossen (Osteo-Pharma, Oss, The Netherlands)]. Genotyping analyses were performed on DNA extracted from tail biopsies with the following oligonucleotides: Lats1, forward 5′-TTGTTGCTGGTGTTGTTTCC-3′; Lats1, reverse 5′-ATGAATGAACCTGAGGCTGC-3′ [generates a 400 bp product for the floxed allele and 250 bp for wild type (WT)]; Lats2, forward 5′-ATCCTAGCACTCAGGAGGCA-3′; and Lats2, reverse 5′-ACACATTCCCCTCCACTGAC-3′ (generates 400 bp product for the floxed allele and 250 bp for WT). The PCR conditions were as follows: 3 min at 94°C for 1 cycle, 15 s at 94°C, 30 s at 55°C, and 45 s at 72°C for 35 cycles and 10 min at 72°C for 1 cycle. The following separate set of primers and conditions was used to detect the KO (i.e., cre-recombined) alleles: Lats1, forward 5′-AGGATGTAGTGAAGGCGTGTAAC-3′; Lats1, reverse 5′-AGACCTCGTCGCACAGAATG-3′ (generates a product of 231 bp); Lats2, forward 5′-CTATCGCTAGGCTGTTCCCAC-3′; and Lats2, reverse 5′-CTGAGCAACGACTCCAGGAAC-3′ (generates a product of 258 bp). The PCR conditions were as follows: 3 min at 94°C for 1 cycle, 15 s at 94°C, 30 s at 56°C, 45 s at 72°C for 35 cycles, and 10 min at 72°C for 1 cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee and conformed to the International Guiding Principles for Biomedical Research Involving Animals.
Breeding trial
Six-week-old Lats1flox/flox;CYP19-cre, Lats2flox/flox;CYP19-cre, and Lats1flox/flox;Lats2flox/flox;CYP19-cre female mice and control littermates (Lats1flox/flox, Lats2flox/flox, and Lats1flox/flox/Lats2flox/flox) were placed in cages with 6-wk-old C57BL/6J males for 6 mo. Cages were monitored daily to record intervals between litters, as well as litter sizes at birth and at weaning. Males were removed after 6 mo and the experiment concluded 22 d later (to allow for any potential final litter).
Tissue collection
Ovaries were collected from 6-d-, 10-d-, 3-wk-, 1-mo-, 2-mo-, 3-mo-, 4-mo-, and 5-mo-old Lats1flox/flox;Lats2flox/flox;CYP19-cre female mice and control littermates and either: 1) flash frozen followed by homogenization for Western blot, quantitative RT-PCR (qRT-PCR), or chromatin immunoprecipitation (ChIP)–quantitative PCR (qPCR) analyses, 2) fixed in 10% formalin and embedded in paraffin for histopathologic analyses or immunohistochemistry (IHC), or 3) embedded in optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA, USA) and frozen for alkaline phosphatase (ALPL) staining. Histopathologic analyses were performed on 6-d (n = 3), 10-d (n = 4), 3-wk (n = 2), 1-mo (n = 5), 2-mo (n = 3), 3-mo (n = 4), 4-mo (n = 5), and 5-mo (n = 4) ovaries (1 ovary/Lats1flox/flox;Lats2flox/flox;CYP19-cre mouse), respectively. Bromodeoxyuridine (BrdU; B5002; MilliporeSigma, Burlington, MA, USA) was reconstituted in sterile saline and administered at 100 mg/kg, i.p. 3 hours prior to tissue collection.
Cell culture
Granulosa cells from Lats1flox/flox, Lats2flox/flox, and Lats1flox/flox;Lats2flox/flox mice primed with 5 IU eCG, i.p. (Folligon; Merck, Darmstadt, Germany) for 44–48 h were seeded onto 96-well plates (0.5 ovaries/well) in Minimum Essential Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with sodium pyruvate (0.25 mM; Thermo Fisher Scientific), l-glutamine (3 mM; Wisent, Saint-Jean-Baptiste, QC, Canada), Pen-Strep (Wisent), and 2% fetal bovine serum (Wisent) for 4 h before infection with either Ad5-cytomegalovirus (CMV)–enhanced green fluorescent protein (Ad-eGFP; control) or Ad5-CMV-Cre-GFP (cre-expressing adenovirus; Ad-cre; Vector Development Laboratory, Baylor College of Medicine, Houston, TX, USA) for 18, 24, or 30 h at a multiplicity of infection of 50. Cells were flash frozen for subsequent analyses by Western blot, qRT-PCR, ChIP-qPCR, and microarray. Alternatively, after 18 h of infection, cells were serum starved for 2 h before treatment with human luteinizing hormone (National Hormone and Peptide Program, Los Angeles Biomedical Research Institute, Los Angeles, CA, USA) at 50 ng/ml for 2 h and flash frozen for subsequent qRT-PCR analyses.
Real-time RT-PCR and microarray analyses
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions. RNA was reverse transcribed using the SuperScript Vilo cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Real-time qPCR was done with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) using the CFX96 Touch Real-Time PCR Detection System and C1000 Touch Thermal Cycler (Bio-Rad). PCR reactions consisted of 2.3 μl of H2O, 6 pM of each forward and reverse gene-specific primer, and 7.5 μl of SYBR Green Supermix. The thermal cycling program consisted of 3 min at 95°C once, 45 s at 95°C, 30 s at 60°C, and 15 s at 72°C for 39 cycles. Relative mRNA levels were determined using Bio-Rad CFX Manager software, with the mathematical model according to Pfaffl (32) and using Rpl19 as the housekeeping gene. Primer sequences for specific genes can be found in Supplemental Table S1. The specificity of all primer pairs was confirmed by sequencing of the PCR products.
Total RNA derived from Lats1flox/flox;Lats2flox/flox granulosa cells infected for 12 or 30 h with either Ad-eGFP or Ad-cre was performed in duplicate for microarray analyses. Mouse Clariom S Assay (Thermo Fisher Scientific) was used and all steps were performed by the McGill University and Génome Québec Innovation Centre (Montréal, QC, Canada). Data was preprocessed using the Affymetrix Gene Expression Console software (Thermo Fisher Scientific), differential analysis of gene expression was performed by the R package limma followed by a Student’s t test. The Canadian Centre for Computational Genomics assisted with data analysis. Functional annotation was performed using the online tool Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/) Bioinformatics Resources v.6.8 (33). A value of P < 0.05 and a 2-fold change cutoff were used to identify differentially expressed genes.
IHC
IHC analyses were performed on formalin-fixed, paraffin-embedded, 3-μm ovarian sections. Sections were probed with the primary antibodies listed in Supplemental Table S2. A Vectastain Elite ABC HRP (horseradish peroxidase) Kit (PK-6101; Vector Laboratories, Burlingame, CA, USA) was used as directed by the manufacturer, followed by staining using the DAB (3,3'-diaminobenzidine) Peroxidase (HRP) Substrate Kit (SK-4100; Vector Laboratories), and counterstaining with hematoxylin before mounting. For mouse primary antibodies, the Mouse on Mouse (M.O.M.) Detection Kit (PK-2200; Vector Laboratories) was used according to the manufacturer’s instructions. IHC for S100 was performed using the Lab Vision Autostainer (Thermo Fisher Scientific) and the slides were counterstained with Congo Red (BioGenex, Fremont, CA, USA).
ALPL staining
ALPL staining was performed on 4-μm ovarian sections embedded in optimal cutting temperature compound. Slides were fixed in 0.2% gluteraldehyde in cold PBS for 10 min, washed in ALPL buffer twice for 5 min [100 mM Tris-HCl (pH 9.5), 100 mM NaCl, 10 mM MgCl2], and then incubated in BCIP/NBT solution [100 mM Tris-HCl (pH 9.5), 100 mM NaCl, 50 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 337 µg/ml 4-Nitro blue tetrazolium chloride, and 175 µg/ml 5-bromo-4-chloro-3-indolyl phosphate; MilliporeSigma] for 15 min. Slides were counterstained with Nuclear Fast Red (5% aluminum sulfate, 0.1% Nuclear Fast Red; 7602-8; MilliporeSigma) for 5 min followed by standard dehydration steps.
Immunoblotting
Frozen granulosa cells (as previously described) or homogenized whole ovaries were lysed in SDS loading buffer, resolved on 10% SDS-polyacrylamide gels, and transferred onto Immobilon-P PVDF Membrane (IPVH00010; MilliporeSigma). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS), and sequentially probed with the antibodies listed in Supplemental Table S2 diluted in 5% bovine serum albumin in TBS (ALB001; Bioshop Canada, Burlington, ON, Canada) overnight at 4°C or β-actin diluted in 5% milk in TBS for 1 h at room temperature. Membranes were then probed with anti-rabbit IgG HRP Conjugate (W401B; Promega, Madison, WI, USA) diluted in 5% milk in TBS for 1 h at room temperature. Immunosignal was detected with Immobilon Western Chemiluminescent HRP Substrate (WBKLS0500; MilliporeSigma). The images were captured with the ChemiDoc MP Imaging System (Bio-Rad), and analyzed with Image Lab Software v.5.0 (Bio-Rad).
ChIP-qPCR
ChIP experiments were performed using either frozen granulosa cells (as previously described) or whole ovaries, as previously described in Svotelis et al. (34), with the exception of using magnetic dynabeads for immunoprecipitation (10002D; Thermo Fisher Scientific). For ovaries, tissues were minced and cross-linked with formaldehyde 1.1% at 37°C with agitation. The cross-linking reaction was stopped by adding 0.125 M of glycine for 10 min at 37°C with agitation followed by 2 washes with cold PBS. Cross-linked ovaries were ground with a dounce homogenizer in SDS lysis buffer. ChIP experiments were performed using 1 µg of TAZ antibody for ovaries, 1 µg of YAP antibody for granulosa cells (listed in Supplemental Table S2), or no antibody as background control. qPCR assays were performed on immunoprecipitated DNA and quantified with a standard curve derived from total DNA (input). Background levels were eliminated by subtracting the amplification levels of samples without antibody from the immunoprecipitated results. Specific primers used for the amplification of bone γ-carboxyglutamate protein (Bglap) and sex-determining region Y box (Sox)9 genes are listed in Supplemental Table S3, including control regions to determine the YAP or TAZ enrichment within the genomic environment. Background results were brought to 0.01% of input for TAZ enrichment and 0.1% of input for YAP enrichment. Data were normalized on the enrichment of Bglap +1650b primers for Bglap coverage in ovary samples or Sox9 −625b primers for Sox9 coverage in granulosa cells samples.
Steroid hormone measurements
Serum was collected from 3-wk-, 2-mo-, and 4-mo-old adult mice. Serum E2 (Calbiotech, El Cajon, CA, USA) and progesterone (IBL USA Shipping, Bayonne, NJ, USA) levels were measured by ELISA. Serum follicle-stimulating hormone and luteinizing hormone (LH) levels were determined by multiplex testing (MilliporeSigma) and radioimmunoassay (in-house protocol). All assays were performed by the Ligand Assay and Analysis Core at the University of Virginia (Charlottesville, VA, USA).
Statistical analyses
All experiments were performed in triplicate. Data are presented as means ± sem. Effects of Lats1 and 2 knockdown on LH responsiveness were analyzed by 1-way ANOVA followed by Tukey’s posttest to identify differences between groups. Effects of Lats1 and 2 ablation on ovarian size, serum hormone levels, gene expression, and normalized enrichment on promoters were analyzed by a Student’s t test. A value of P ≤ 0.05 was considered statistically significant. Analyses were performed using Prism software v.6.01 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Ovarian tissue overgrowth and infertility in Lats1flox/flox;Lats2flox/flox;CYP19-cre mice
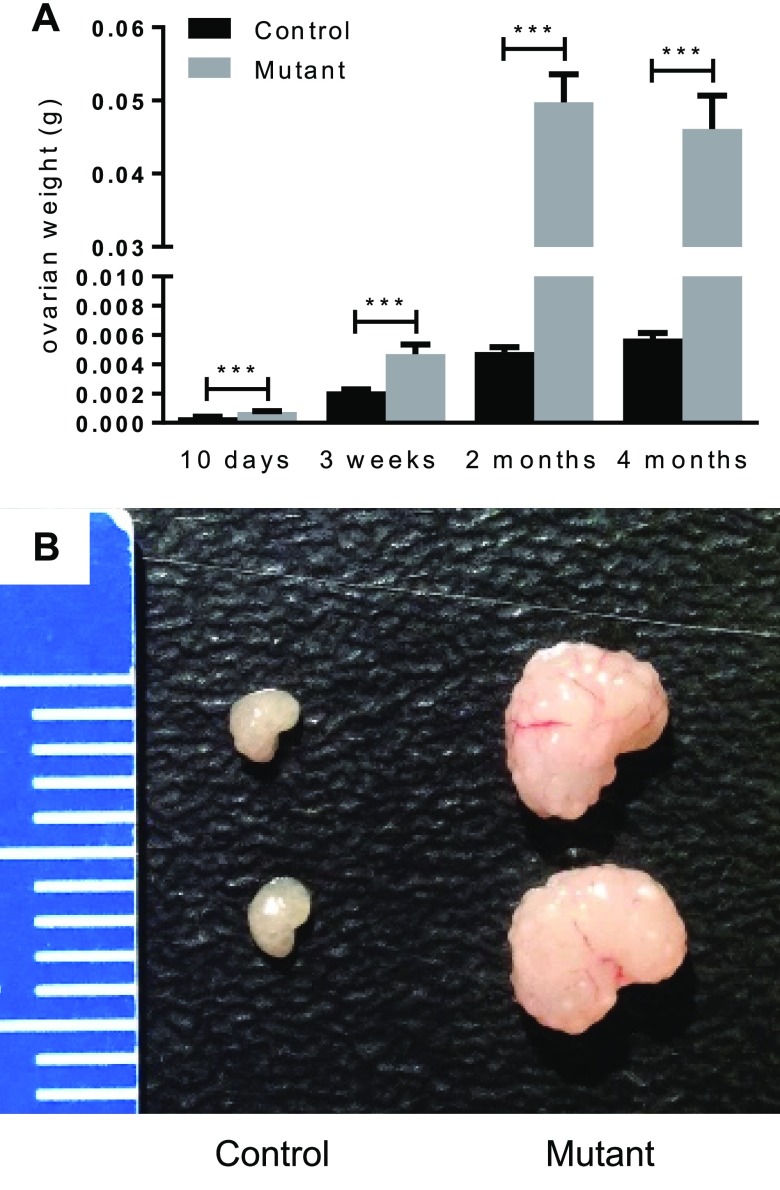
To study the roles of Lats1 and 2 in the ovary, granulosa cell–specific conditional KO mice were generated by mating strains bearing Lats1- and Lats2-floxed alleles to the CYP19-cre strain (35, 36). Both Lats1flox/flox;CYP19-cre and Lats2flox/flox;CYP19-cre females were fertile and had no obvious ovarian defects (unpublished results). Hypothesizing that the lack of phenotypic abnormalities in these mice was caused by functional redundancy of Lats1 and 2 in granulosa cells, we generated Lats1flox/flox;Lats2flox/flox;CYP19-cre mice to inactivate both genes concomitantly. To assess fertility, 5 Lats1flox/flox;Lats2flox/flox;CYP19-cre females were placed in 6-mo breeding trials with WT males. One of these mice produced 2 small litters, whereas the others were sterile (Table 1). Examination of the ovaries of Lats1flox/flox;Lats2flox/flox;CYP19-cre mice at different ages showed a dysregulation of postnatal development, with growth occurring at an accelerated pace and ovarian weights attaining ∼10-fold that of controls by 2 mo of age but not increasing significantly thereafter (Fig. 1A). The ovaries of adult Lats1flox/flox;Lats2flox/flox;CYP19-cre mice had a distinctive lobular appearance and hardened consistency (Fig. 1B). Analyses of serum hormone levels in 4-mo-old mice showed that progesterone levels were ∼6-fold lower in Lats1flox/flox;Lats2flox/flox;CYP19-cre animals relative to controls (2.509 ± 0.7327 vs. 15.10 ± 4.093 ng/ml; means ± sem; n = 8/genotype; P < 0.01), and that E2 levels were beneath the radioimmunoassay detection threshold. Conversely, vastly increased circulating follicle-stimulating hormone and LH levels were found in adult Lats1flox/flox;Lats2flox/flox;CYP19-cre females (Supplemental Fig. S1), together suggesting that ovarian failure had occurred.
TABLE 1.
Breeding trials
| Variable (n) | Lats1flox/flox;Lats2flox/flox | Lats1flox/flox;Lats2flox/flox;CYP19-cre |
|---|---|---|
| Mating pairs | 7 | 5 |
| Total litters | 52 | 2 |
| Total pups | 471 | 12 |
Figure 1.
Lats1flox/flox;Lats2flox/flox;CYP19-cre ovaries. Ovarian weights of Lats1flox/flox;Lats2flox/flox (control) and Lats1flox/flox;Lats2flox/flox;CYP19-cre (mutant) (n = 16 ovaries/time point) (A) with representative images (B). Data are means ± sem. ***P ≤ 0.001 (statistically significant differences between groups). Ruler graduations are in millimeters.
Loss of Lats1 and 2 causes loss of granulosa cell identity, possible EMT, and transdifferentiation into Sertoli-like cells
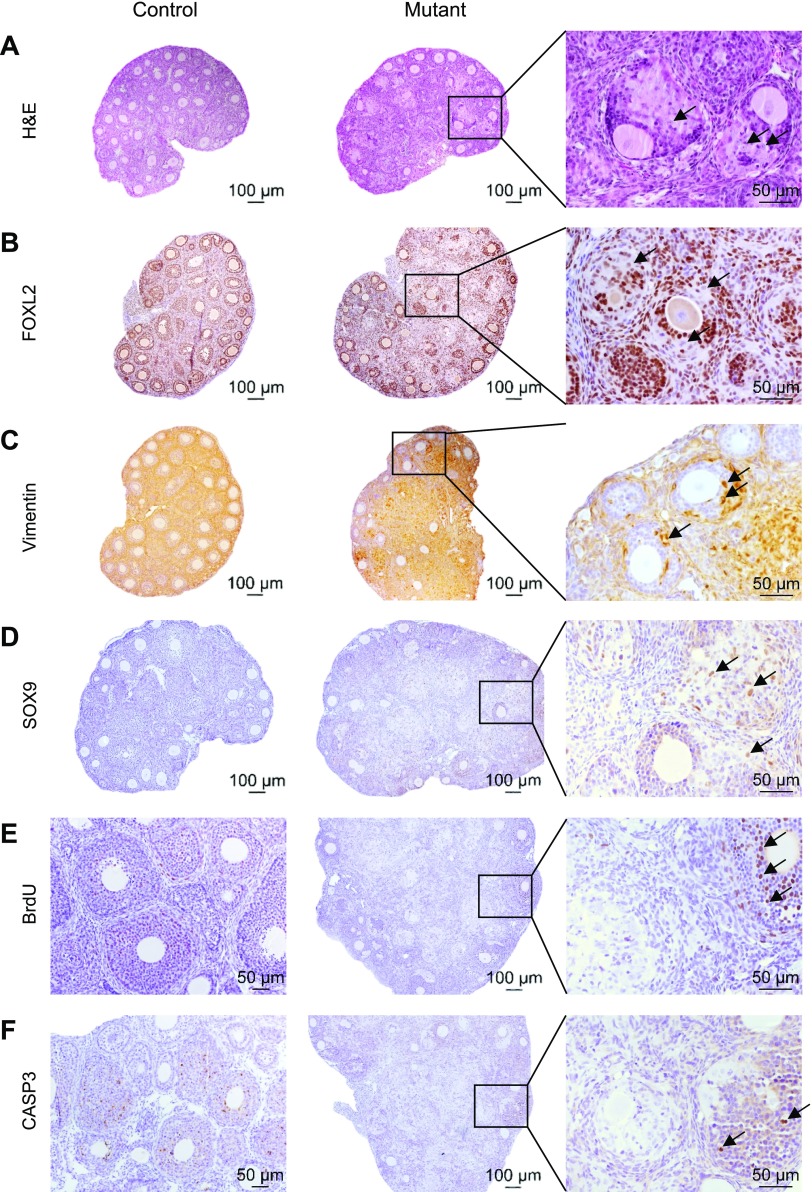
Histopathologic analyses of ovaries from 6-d-old Lats1flox/flox;Lats2flox/flox;CYP19-cre mice showed no differences relative to controls (unpublished results). However, at 10 d of age [corresponding to the earliest reported ovarian expression of the CYP19-cre transgene (37)], striking abnormalities were observed. Many follicles were found to contain a new population of cells featuring large nuclei, prominent nucleoli, and abundant cytoplasm (Fig. 2A). These changes occurred mostly in follicles located in the ovarian medulla, where the first wave of follicle development normally occurs (38). IHC analyses revealed that the new cell type did not express the granulosa cell marker forkhead box L2 (FOXL2) (Fig. 2B). Rather, it expressed the mesenchymal cell marker vimentin (Fig. 2C). Interestingly, a subpopulation of the cells was also positive for the Sertoli cell marker SOX9 (Fig. 2D). BrdU incorporation assays demonstrated that these cells were not proliferative (Fig. 2E), and caspase-3 (CASP3) IHC (Fig. 2F) analyses showed that they never underwent apoptosis, unlike the adjacent granulosa cells. Together, these results suggest that the granulosa cells in the ovaries of Lats1flox/flox;Lats2flox/flox;CYP19-cre mice undergo EMT and transdifferentiate into a heterogeneous cell population. Increased ovary size in these mice was apparently caused by the larger size of the transdifferentiated cells relative to granulosa cells and to their evasion of apoptosis but was not caused by increased cell proliferation.
Figure 2.
Loss of Lats1 and 2 causes loss of granulosa cell identity, possible EMT, and transdifferentiation into Sertoli-like cells. A) Representative images of Lats1flox/flox;Lats2flox/flox (control) and Lats1flox/flox;Lats2flox/flox; CYP19-cre (mutant) ovaries at 10 days postpartum (dpp). Inset: follicles containing a new cell type (arrows). B) FOXL2 IHC. Inset: the new cells are FOXL2-negative (arrows). C) Vimentin IHC. Inset: the new cells are vimentin-positive (arrows). D) SOX9 IHC. Inset: occasional new cells are SOX9-positive (arrows). E) BrdU incorporation assay on ovaries at 21 dpp; arrows indicate normal granulosa cells staining positive for BrdU. F) Cleaved CASP3 IHC at 21 dpp; arrows indicate normal granulosa cells staining positive for CASP3. H&E, hematoxylin and eosin.
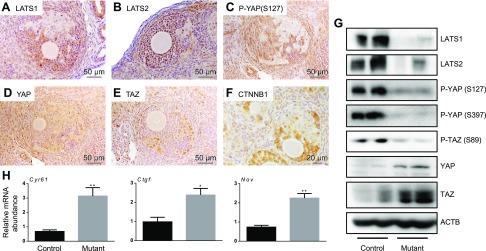
To determine how loss of Lats1 and 2 affected Hippo signaling in the ovaries of Lats1flox/flox;Lats2flox/flox;CYP19-cre mice, IHC and immunoblotting analyses of Hippo pathway effectors and qRT-PCR analyses of Hippo target gene expression were conducted. These analyses confirmed that the transdifferentiated cells do not express Lats1 or 2 (Fig. 3A, B). Loss of Lats1 and 2 resulted in decreased phosphorylation of YAP at Ser127 and consequential increases in YAP (and TAZ) protein levels relative to the adjacent normal-looking granulosa cells (Fig. 3C–E). Although recent reports have suggested that the stability of YAP and TAZ and that of the Wingless-type MMTV integration site family (WNT) pathway effector β-catenin (CTNNB1) are interdependent (39, 40), CTNNB1 levels were found to be decreased in the transdifferentiated cells (Fig. 3F). Similar decreases in LATS1, LATS2, phospho-YAP (both Ser127 and Ser397) and phospho-TAZ (Ser89), as well as increases in total YAP and TAZ levels, were observed by immunoblot analyses of whole ovaries from 4-wk-old Lats1flox/flox;Lats2flox/flox;CYP19-cre mice, relative to age-matched controls (Fig. 3G). The mRNA levels of the YAP and TAZ target genes cysteine-rich 61, connective tissue growth factor (Ctgf), and nephroblastoma overexpressed were also increased in the ovaries of Lats1flox/flox;Lats2flox/flox;CYP19-cre mice (Fig. 3H). These results link the transdifferentiation and EMT-like processes observed in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model to a disruption in the Hippo kinase cascade, with resultant accumulation of YAP and TAZ proteins and transactivation of their target genes.
Figure 3.
Hippo signaling is disrupted in Lats1flox/flox;Lats2flox/flox;CYP19-cre ovaries. Representative images of Lats1flox/flox;Lats2flox/flox;CYP19-cre (mutant) ovaries. A-F)LATS1 IHC of ovarian sections from 21-d-old mutant mice (A), LATS2 (B), phospho-YAP (Ser127) (C), YAP (D), TAZ (E), and CTNNB1 (F). G) Representative immunoblots of whole 4-wk-old control and mutant ovaries show n = 2 ovaries/genotype. H) qRT-PCR was performed on whole ovaries from 2-mo-old control vs. mutant mice to determine mRNA levels of YAP, TAZ, and TEAD target genes (n = 4 ovaries/genotype). Data were normalized to the housekeeping gene Rpl19. Data are means ± sem. *P ≤ 0.05, **P ≤ 0.01 (statistically significant differences between groups).
Multilineage transdifferentiation in the ovaries of Lats1flox/flox;Lats2flox/flox;CYP19-cre mice
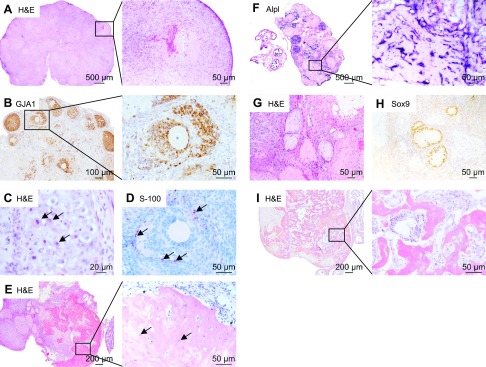
After 10 d of age, transdifferentation progressed throughout the granulosa cell population in Lats1flox/flox;Lats2flox/flox;CYP19-cre mice, leading to ovaries almost entirely devoid of normal follicles. By 1 mo of age, the ovaries were mainly composed of spindle-shaped cells organized into follicle-like structures delineated by basal lamina and with necrotic centers (Fig. 4A). The necrotic material appeared to consist of decaying granulosa cells and oocytes. Gap junction protein α-1 (GJA1) IHC revealed that a broad loss of gap junctions occurred through the transdifferentiation process (Fig. 4B). Because granulosa cells and oocytes rely on extensive gap junction networks for nutrient transport through the (avascular) granulosa cells layer, the remaining (nontransdifferentiated) granulosa cells and oocytes may therefore have degenerated because of nutrient deprivation.
Figure 4.
Lats1flox/flox;Lats2flox/flox;CYP19-cre granulosa cells appear to transdifferentiate into multiple cell lineages. A) Representative image of a Lats1flox/flox;Lats2flox/flox;CYP19-cre (mutant) ovary at 30 dpp. Inset: follicle-like structure containing a necrotic center. B) GJA1 (connexin 43) IHC on ovarian sections from 21-d-old mutant mice. Inset: new cell populations lack the gap junction protein GJA1. C) Representative image of a follicle-like structure containing abundant eosinophilic cytoplasmic granules (arrows) from 3-mo-old mutant mice. D) S-100 IHC on ovarian sections from 30-d-old mutant mice. Occasional cells (of the new cell populations) stain positive for S-100 (arrows). E) Representative image of mutant ovaries at 4 mo. Inset: osteoblasts (asterisk) and osteocytes (arrows) are clearly visible. F) ALPL staining. Inset: osteoblasts and osteocytes stain positive for ALPL. G) Representative image of cord-like structures. H) The cells lining the cord-like structures stain positive for SOX9. I) Representative image of mutant ovaries at 5 mo of age that are almost entirely composed of bone and bone marrow (inset). H&E, hematoxylin and eosin.
By 3 mo of age, a new cell population was occasionally observed within the follicle-like structures, featuring abundant eosinophilic granules in the cytoplasm (Fig. 4C), possibly of a neurosecretory or immune lineage. Furthermore, a subpopulation of cells was found to be positive for S100 (Fig. 4D), a marker of cell types derived from the neural crest. Strikingly, by 4 mo of age, many follicle-like structures composed of spindle-shaped cells were still present but the majority of the ovary was occupied by a partially mineralized osteoid matrix (Fig. 4E). Osteoblasts and osteocytes were clearly visible (Fig. 4E) and were stained positive for ALPL (Fig. 4F). Cord-like structures were often found, consisting of prominent basement membrane lined with large ovoid cells, oriented perpendicular to the basement membrane, with cytoplasmic veils extending into the lumen (Fig. 4G). The latter cells stained positive for SOX9 (Fig. 4H) and the cords were therefore designated as seminiferous tubule-like structures, although they were devoid of germ cells. At 5 mo of age, the ovaries were composed nearly entirely of bone, consisting of osteoblasts, osteoclasts, and bone marrow replete with hematopoietic cells (Fig. 4I).
YAP and TAZ drive the ectopic expression of the osteoblast and Sertoli cell genetic programs in Lats1- and Lats2-depleted granulosa cells
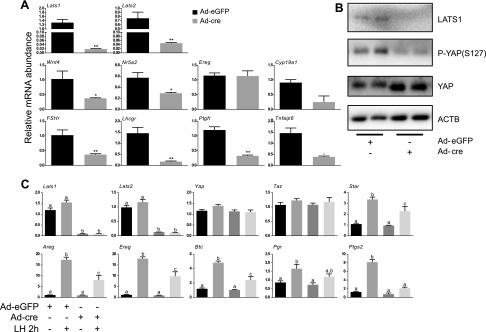
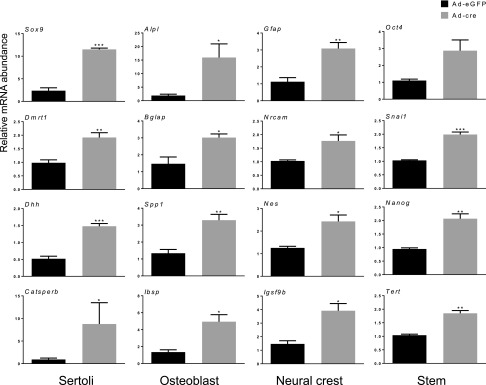
To study the molecular mechanisms underlying the phenotypic changes observed in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model, we developed a primary granulosa cell culture system in which Lats1 and 2 could be inactivated acutely. Granulosa cells isolated from eCG-primed immature Lats1flox/flox;Lats2flox/flox mice were placed in culture and infected with adenoviruses to drive expression of either eGFP (Ad-eGFP, control) or cre (Ad-cre, to recombine the floxed alleles). Ad-cre treatment for 30 h resulted in ∼65- and 15-fold reductions in Lats1 and 2 mRNA levels, respectively, and was accompanied by a loss of LATS1 protein and YAP phosphorylation, along with an increase in total YAP levels (Fig. 5A, B). This was also accompanied by a dramatic loss in the expression of genes associated with granulosa cell differentiation and function, including Wnt4, Fshr, Lhcgr, and Cyp19a1 (Fig. 5A). Loss of granulosa cell function was further evidenced by the blunted response of Lats1- and Lats2-depleted granulosa cells to LH, characterized by a significantly impaired induction of LH target genes such as amphiregulin, epiregulin, betacellulin, prostaglandin-endoperoxide synthase 2, and steroidogenic acute regulatory protein (Fig. 5C). Whereas the LH response was also blunted to some extent in granulosa cells in which Lats1 or 2 alone were depleted (Supplemental Fig. S2A, B), the effect was not nearly as pronounced as when they were depleted concomitantly, confirming the redundancy of Lats1 and 2 function in this context. In addition to the loss of granulosa cell gene expression and function, Lats1- and 2-depleted granulosa cells also had dramatically increased mRNA levels of genes associated with Sertoli cells (Fig. 6). This was also observed, albeit to a lesser extent, in granulosa cells deficient in Lats1 or 2 alone (Supplemental Fig. S2C, D). Lats1- and 2-depleted granulosa cells expressed increased levels of genes associated with osteoblasts and neural cell lineages, as well as markers of stem and progenitor cell types (Fig. 6). These data therefore indicate that the culture system faithfully replicated the granulosa cell transdifferentiation phenotype observed in vivo in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model.
Figure 5.
Knockdown of Lats1 and 2 in vitro causes a loss of granulosa cell identity and function. A) Lats1flox/flox;Lats2flox/flox granulosa cells were infected with either Ad-eGFP or Ad-cre for 30 h and mRNA levels of the indicated genes were determined by qRT-PCR and normalized to the housekeeping gene Rpl19 (n = 3 replicates/treatment). Data are means ± sem. *P ≤ 0.05, **P ≤ 0.01 (statistically significant differences between groups). B) Lats1flox/flox;Lats2flox/flox granulosa cells were infected with either Ad-eGFP or Ad-cre for 24 h and the expression of the indicated proteins was evaluated by immunoblot analysis (n = 2 replicates per treatment are shown). C) Lats1flox/flox;Lats2flox/flox granulosa cells were infected with either Ad-eGFP or Ad-cre for 18 h, followed (or not) by treatment with LH (50 ng/ml) for 2 h, and mRNA levels of the indicated genes were determined by qRT-PCR and normalized to the housekeeping gene Rpl19 (n = 4 replicates/treatment). Data are means ± sem. Different letters (a–c) show statistically significant differences between groups (P ≤ 0.05).
Figure 6.
Knockdown of Lats1 and 2 in granulosa cells in vitro induces transdifferentiation into multiple cell lineages. A) Lats1flox/flox;Lats2flox/flox granulosa cells were infected with either Ad-eGFP or Ad-cre for 30 h and mRNA levels of the indicated genes were determined by qRT-PCR and normalized to the housekeeping gene Rpl19 (n = 3 replicates/treatment). Data are means ± sem. *P ≤ 0.05, **P ≤ 0.01, **P ≤ 0.001 (statistically significant differences between groups).
To gain further insight into the cellular and molecular processes that are altered following Lats1 and 2 depletion, microarray analyses were conducted on granulosa cells using the culture system described above. An increase of 2-fold or greater was found in the expression of 58 transcripts, and a decrease for 101 transcripts, at 12 h posttreatment in Ad-cre–treated cells vs. Ad-eGFP–treated cells (threshold: P < 0.01) (Supplemental Data S1). These numbers increased to 174 and 328, respectively, by 30 h (Supplemental Data S1). Functional analysis of the array data using DAVID (33) notably identified large groups of genes involved in regulating apoptosis and the cell cycle [Supplemental Data S2; selected biological process Gene Ontology (GO) terms from the 30-h data set are presented in Table 2], providing a potential basis for the abrogation of proliferation and apoptosis observed in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model. Also consistent with the in vivo transdifferentiation phenotype, transcripts related to osteoblastic differentiation, male and female gonad development, ossification, and spermatogenesis were identified by the GO term analysis (Table 2). Unexpectedly, however, groups of genes related to adrenal gland, kidney, and liver development and keratinocyte differentiation were also identified, suggesting that loss of Lats1 and 2 affected the expression of the genetic programs of a number of additional cell lineages (Table 2).
TABLE 2.
Selected biological process GO terms from the microarray data set
| Biological process | Count | P | Fold enrichment |
|---|---|---|---|
| Cell-cell adhesion | 19 | 1.37E−05 | 3.39 |
| G1/S transition of mitotic cell cycle | 10 | 7.89E−05 | 5.47 |
| Negative regulation of apoptotic process | 34 | 1.78E−04 | 2.02 |
| Osteoblast differentiation | 12 | 6.32E−04 | 3.51 |
| Negative regulation of cell proliferation | 24 | 1.24E−03 | 2.1 |
| Cell adhesion | 28 | 1.25E−03 | 1.96 |
| Adrenal gland development | 6 | 1.44E−03 | 7.01 |
| Regulation of cell cycle | 11 | 1.99E−03 | 3.27 |
| Positive regulation of apoptotic process | 21 | 2.50E−03 | 2.11 |
| Angiogenesis | 15 | 1.24E−02 | 2.11 |
| Male gonad development | 9 | 1.69E−02 | 2.75 |
| Kidney development | 10 | 1.85E−02 | 2.51 |
| Blood vessel morphogenesis | 5 | 1.89E−02 | 4.84 |
| G2/M transition of mitotic cell cycle | 5 | 1.89E−02 | 4.84 |
| Ossification | 8 | 2.45E−02 | 2.79 |
| Hippo signaling | 4 | 2.58E−02 | 6.16 |
| Positive regulation of cell proliferation | 25 | 3.25E−02 | 1.56 |
| Spermatogenesis | 20 | 3.68E−02 | 1.64 |
| Keratinocyte differentiation | 7 | 3.75E−02 | 2.82 |
| Liver development | 8 | 4.77E−02 | 2.42 |
| Female gonad development | 4 | 4.83E−02 | 4.84 |
Microarray analyses were performed on Lats1flox/flox;Lats2 flox/flox granulosa cells infected with Ad-eGFP or Ad-cre for 30 h.
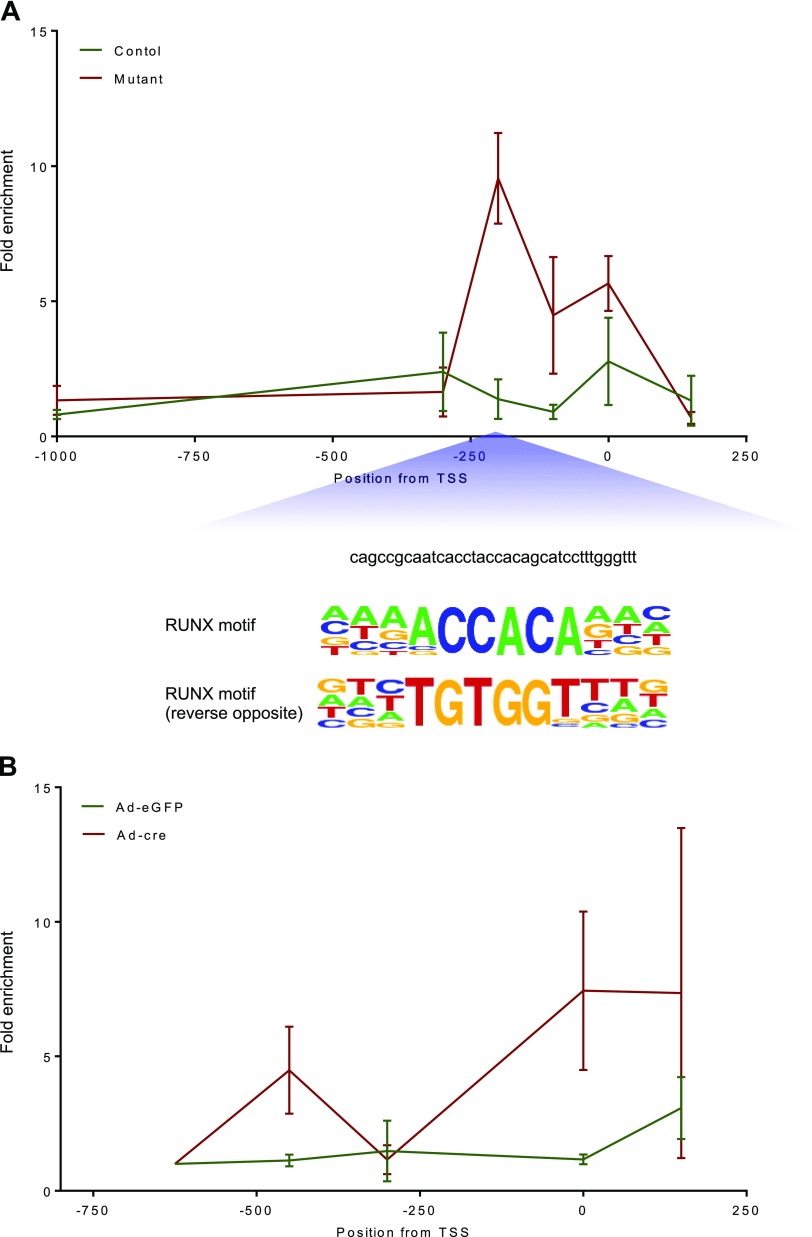
To further define how loss of Lats1 and 2 induces transdifferentiation, we examined the recruitment of YAP and TAZ to the proximal promoters of Sox9 and Bglap (osteocalcin), 2 key genes respectively involved in male sex determination and osteogenesis (41, 42) that have been previously described as direct transcriptional targets of YAP (Sox9) and TAZ (Bglap) (43, 44). ChIP-qPCR analyses comparing ovaries from 2-mo-old Lats1flox/flox;Lats2flox/flox;CYP19-cre and control mice showed markedly enhanced recruitment of TAZ to the Bglap promoter in the mutant mice (Fig. 7A). This effect was maximal ∼200 base pairs upstream of the transcriptional start site in the vicinity of a consensus RUNX-binding motif. For Sox9, ChIP analyses were performed on primary cultured granulosa cells from Lats1flox/flox;Lats2flox/flox mice treated with adenoviruses as described above. Ad-cre treatment (i.e., Lats1 and 2 ablation) resulted in enhanced recruitment of YAP to the Sox9 proximal promoter, particularly near the transcriptional start site (Fig. 7B). However, in silico analyses failed to identify binding motifs for transcription factors known to bind YAP in this region. Together, these data suggest that loss of Lats1 and 2 drives transdifferentiation by increasing YAP- and TAZ-promoter binding activity, leading to the direct transcriptional up-regulation of key genes involved in cell fate determination.
Figure 7.
Bglap and Sox9 ChIP analyses. A) ChIP-qPCR was performed using ovaries from Lats1flox/flox;Lats2flox/flox;CYP19-cre (mutant) or Lats1flox/flox;Lats2flox/flox (control) mice evaluating the enrichment of TAZ on the Bglap promoter. B) ChIP-qPCR was performed using Lats1flox/flox;Lats2flox/flox granulosa cells infected with either Ad-eGFP or Ad-cre for 30 h evaluating the enrichment of YAP on the Sox9 promoter. Data are means ± sem (n = 3 replicates/experiment).
DISCUSSION
Several recent reports have begun to define the multipotency and stem-like characteristics of ovarian granulosa cells (45). In primary culture, granulosa cells have been reported to gradually lose their characteristic gene expression patterns and functions (e.g., steroidogenesis), concomitantly with increases in the expression of stem cell markers (46, 47). Specific culture conditions and treatments have then been used to induce these cells to transdifferentiate into neurons, chondrocytes, and osteoblasts (46, 48, 49). Granulosa cell transdifferentiation can also be induced in vivo following specific genetic modifications. We have reported that ossification and expression of neuronal and neurosecretory cell markers occurs in the ovaries of mice bearing an activating mutation in Ctnnb1 targeted to granulosa cells (50, 51). Likewise, targeted inactivation of FoxL2 (52) or ectopic expression of doublesex and mab-3 related transcription factor 1 (Dmrt1) (53) causes granulosa cells to switch to the Sertoli cell fate and form seminiferous tubules. Despite its exceptional nature, the molecular basis for this apparent cell fate plasticity of granulosa cells remains largely unknown. In this report, we show that the loss of the core Hippo kinases Lats1 and 2 caused granulosa cells to rapidly lose their morphologic characteristics, functions, and gene expression profiles, and increase the expression of stem and progenitor cell markers. Concomitantly, a multilineage transdifferentiation process occurred, leading to the appearance of cells with neural, neurosecretory, Sertoli, and osteoblast characteristics. Microarray analyses suggested that additional lineages may also have been generated early in the transdifferentiation process, but we were unable to unequivocally identify other cell types, possibly because they were rapidly overwhelmed by the dominant osteoblast lineage. Together, our findings indicate that Hippo is a key regulator of granulosa cell plasticity and that Lats1 and 2 act in a redundant manner to maintain granulosa cell fate.
The embryonic process of sex determination is complex and depends upon a fine balance between the expression of female- vs. male-specific genes to cause bipotential precursor cells to adopt either the granulosa or Sertoli cell fate. In XX mammals, female-specific WNT4/CTNNB1/R-spondin 1 signaling and FOXL2 act to suppress Sox9 expression, resulting in ovarian development (54). In XY individuals, Sox9 and Dmrt1 suppress the female genetic program, resulting in Sertoli cell differentiation and testis development. Importantly, the expression of these sex-specific genetic programs must be maintained even throughout adulthood or phenotypic sex reversal (i.e., granulosa and Sertoli transdifferentiation) occurs (52, 53, 55). Our finding of transdifferentiation into Sertoli-like cells in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model was unexpected, because little evidence existed beforehand to suggest that Hippo could be involved in sex determination. The only previous report to this effect showed that male mice with conditional deletion of Yap and Taz targeted to Sertoli cells have decreased testicular expression of genes associated with male sex determination (desert hedgehog, Dmrt1, and Sox9) and the female sex-differentiation gene Wnt4 was up-regulated, however sex reversal did not occur in this model (56). Combined with the latter report, our findings therefore identify YAP and TAZ as positive regulators of Sox9 expression. Whether this indicates that Hippo signaling plays a role during the embryonic process of sex determination or if it is only required for postnatal maintenance of granulosa cell fate will be grounds for further study. The mechanism whereby YAP and TAZ regulate Sox9 will also require further clarification. Our ChIP analyses suggest that YAP may induce Sox9 transcriptional activity directly by binding the Sox9 promoter via unknown transcription factors. However, this does not preclude additional mechanisms of YAP and TAZ action in the context of transdifferentiation into Sertoli-like cells. For instance, suppression of FoxL2 transcriptional activity by YAP and TAZ could also be involved in the induction of Sox9 expression. Interestingly, CTNNB1 has recently been shown to be a positive transcriptional regulator of FoxL2 (57), and CTNNB1 expression is lost in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model, suggesting an additional mechanism that may contribute to the transdifferentiation phenotype. Whether and how YAP and TAZ may contribute to the loss of CTNNB1 in this model also remains to be determined.
Because a previous study had shown that TAZ-mediated induction of Bglap via RUNX2 drives osteoblast differentiation in mesenchymal stem cells (44), we examined potential direct regulation of Bglap by TAZ in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model. Our ChIP analyses revealed enhanced recruitment of TAZ to the Bglap promoter, particularly in a region containing a previously characterized (58) consensus RUNX-binding motif. RUNX2 is a well-established binding partner for YAP and TAZ and is a critical mediator of osteogenesis (59). We therefore conclude direct transcriptional induction of Bglap by TAZ and RUNX2 to be a major mechanism driving osteoblast transdifferentiation following loss of Hippo signaling. However, additional mechanisms are certainly involved. For instance, snail family transcriptional repressor (Snai)1 and Snai2 represent important mediators of osteogenesis and encode the zinc finger transcription factors Snail1 and Snail2 (Slug). YAP and TAZ have been reported to form a complex with SNAIL and SLUG and together bind RUNX2 (60). Deletion of both Snai1 and Snai2 was shown to inhibit the expression of RUNX2 target genes that are essential for osteogenesis (60). In our study, Snai1 was up-regulated in vitro following the loss of Lats1 and 2, further supporting the notion that osteoblast transdifferentiation could be mediated in part by YAP, TAZ, SNAIL, and SLUG enhancement of RUNX2-mediated transcription of osteogenic genes. Another potential driver of osteogenesis is the YAP, TAZ, and TEAD target gene Ctgf, which encodes the matricellular protein CTGF. CTGF is essential for bone formation, as evidenced by Ctgf-KO mice, which develop skeletal deformities and have reduced ossification (61). In our study, Ctgf expression was markedly increased both in vitro following Lats1 and 2 inactivation and in vivo in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model. The contributions of these (and other) potential mechanisms to granulosa-to-osteoblast transdifferentiation will be grounds for further study.
The ovaries of Lats1flox/flox;Lats2flox/flox;CYP19-cre mice were also found to contain cell populations that express markers of cell types derived from the neural crest, an embryonic structure which gives rise to most of the peripheral nervous system (62). Relatively few studies have studied Hippo signaling in the context of nervous system development. Of note, YAP was found to be required for astrocytic differentiation (63), whereas the YAP paralog Yorkie was found to dictate the terminal differentiation of neuroblasts (64). In addition to its roles in bone tissue, Ctgf is also expressed during embryonic brain development and may play a role in the process whereby mesenchymal stem-like cells differentiate into neural crest cell types (65). We speculate that Ctgf may therefore be a critical component of the mechanism through which the loss of Hippo signaling results in granulosa cell transdifferentiation into multiple lineages.
EMT occurs over the course of development in wound healing and in cancer. It involves the loss of epithelial cell characteristics, such as cell polarity and cell adhesion, and the acquisition of mesenchymal cell characteristics, such as spindle shape, migration, and stemness (66). In the Lats1flox/flox;Lats2flox/flox;CYP19-cre model, a subset of Lats1- and 2-null granulosa cells appears to transition into vimentin-positive, spindle-shaped, mesenchymal stem cell–like cells. Both Yap and Taz have been implicated in EMT (67, 68). Indeed, YAP forms a complex with the TGF-β family–signaling effectors Mothers Against Decapentaplegic homolog (SMAD)2, 3, and 4, which are required for the up-regulation of the transcription factors Snai1, Snai2, and Twist, which in turn drive EMT (68). We therefore suspect that the accumulation of YAP and TAZ in Lats1 and 2-null granulosa cells is responsible for promoting an EMT-like process by a mechanism that could involve SMAD2, 3, and 4 signaling. Although the relevance of this process to the transdifferentiation phenotype observed in the ovaries of Lats1flox/flox;Lats2flox/flox;CYP19-cre mice remains to be determined, EMT is thought to enable transdifferentiation in certain contexts by converting differentiated epithelial cells into pluripotent mesenchymal precursors (69). That this process should precede transdifferentiation would seem compatible with several observations made in the Lats1flox/flox;Lats2flox/flox;CYP19-cre model, such as the appearance of a spindle-shaped cell population prior to osteoblast transdifferentiation.
In summary, this is the first report demonstrating that disruption of the Hippo pathway can result in apparent phenotypic sex reversal. Lats1- and 2-null granulosa cells further transdifferentiated into osteoblasts and possibly other cell lineages, illustrating a critical role for LATS kinases as barriers to reprogramming. This reprogramming appears to be driven by an accumulation of YAP and TAZ, which act via various transcription factors to drive the ectopic activation of the genetic programs of multiple cell lineages. These findings invite future investigation of the potential roles of Hippo in the physiologic process of sex determination during embryogenesis and may contribute to advancing fields such as stem cell biology and regenerative medicine.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Claude Paquet, Philippe Godin, and Meggie Girard (all from Université de Montréal) for technical support. The authors thank Jan Gossen (Osteo-Pharma, Oss, The Netherlands) for CYP19-cre mice. The authors thank McGill University and Génome Québec Innovation Centre (Montréal, QC, Canada) and the Canadian Center for Computational Genomics (C3G; Montréal, QC, Canada) for generating and analyzing microarray data. C3G is a node of the Canadian Genomic Innovation Network and is supported by the Canadian government through Genome Canada. The authors thank Dagmar Wilhelm (University of Melbourne, Parkville, VIC, Australia) for the FOXL2 antibody. The authors thank the Ligand Assay and Analysis Core at the University of Virginia (Charlottesville, VA, USA) for the hormonal measurements. This work was supported by Canadian Institutes of Health Research (CIHR) operating Grant MOP-142445 (to D.B.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by U.S. National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development Specialized Cooperative Centers Program in Reproduction Research (SCCPRR) Grant U54-HD28934. M.T. was supported by a doctoral research award from the Fonds de Recherche du Québec–Santé (FRQS). The authors declare no conflicts of interest.
Glossary
- Ad-cre
cre-expressing adenovirus
- Ad-eGFP
Ad5-cytomegalovirus–enhanced green fluorescent protein
- ALPL
alkaline phosphatase
- BGLAP
bone γ-carboxyglutamate protein
- BrdU
bromodeoxyuridine
- CASP3
caspase-3
- ChIP
chromatin immunoprecipitation
- CTGF
connective tissue growth factor
- CTNNB1
β-catenin
- CYP
cytochrome P450
- DMRT1
doublesex and mab-3 related transcription factor 1
- EMT
epithelial-to-mesenchymal transition
- FOXL2
forkhead box L2
- GJA1
gap junction protein α-1
- GO
Gene Ontology
- HRP
horseradish peroxidase
- IHC
immunohistochemistry
- KO
knockout
- LATS
large tumor suppressor
- LH
luteinizing hormone
- MST
mammalian STE20-like protein kinase
- phospho
phosphorylated
- qPCR
quantitative PCR
- qRT-PCR
quantitative RT-PCR
- RUNX
runt-related transcription factor
- Slug
SNAI2
- SNAI
snail family transcriptional repressor
- SOX
sex-determining region Y box
- TAZ
transcriptional coactivator with PDZ-binding motif
- TBS
Tris-buffered saline
- TEAD
TEA domain transcription factor
- WNT
Wingless-type MMTV integration site family
- WT
wild type
- YAP
Yes-associated protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Tsoi performed murine work, acquisition of data, analysis and interpretation of data, and drafting of the manuscript; M. Morin and C. Rico performed acquisition, analysis, and interpretation of data; and R. L. Johnson, M. Paquet, N. Gévry, and D. Boerboom created the study concept and design, performed analysis and interpretation of data, drafting and critical revision of the manuscript for important intellectual content, and study supervision.
REFERENCES
- 1.Zhao B., Li L., Lei Q., Guan K. L. (2010) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 24, 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan D. (2010) The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccolo S., Dupont S., Cordenonsi M. (2014) The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 [DOI] [PubMed] [Google Scholar]
- 4.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 [DOI] [PubMed] [Google Scholar]
- 5.Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., Guan K. L. (2007) Inactivation of YAP oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callus B. A., Verhagen A. M., Vaux D. L. (2006) Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 273, 4264–4276 [DOI] [PubMed] [Google Scholar]
- 7.Praskova M., Xia F., Avruch J. (2008) MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 18, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan E. H., Nousiainen M., Chalamalasetty R. B., Schäfer A., Nigg E. A., Silljé H. H. (2005) The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24, 2076–2086 [DOI] [PubMed] [Google Scholar]
- 9.Pearce L. R., Komander D., Alessi D. R. (2010) The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 10.Liu C. Y., Zha Z. Y., Zhou X., Zhang H., Huang W., Zhao D., Li T., Chan S. W., Lim C. J., Hong W., Zhao S., Xiong Y., Lei Q. Y., Guan K. L. (2010) The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFbeta-TrCP E3 ligase. J. Biol. Chem. 285, 37159–37169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B., Li L., Tumaneng K., Wang C. Y., Guan K. L. (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 24, 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu S., Totty N. F., Irwin M. S., Sudol M., Downward J. (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 11, 11–23 [DOI] [PubMed] [Google Scholar]
- 13.Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S. A., Gayyed M. F., Anders R. A., Maitra A., Pan D. (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassilev A., Kaneko K. J., Shu H., Zhao Y., DePamphilis M. L. (2001) TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J. D., Wang C. Y., Chinnaiyan A. M., Lai Z. C., Guan K. L. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitolo M. I., Anglin I. E., Mahoney W. M., Jr., Renoud K. J., Gartenhaus R. B., Bachman K. E., Passaniti A. (2007) The RUNX2 transcription factor cooperates with the YES-associated protein, YAP65, to promote cell transformation. Cancer Biol. Ther. 6, 856–863 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki H. (2017) Roles and regulations of Hippo signaling during preimplantation mouse development. Dev. Growth Differ. 59, 12–20 [DOI] [PubMed] [Google Scholar]
- 18.Nishioka N., Inoue K., Adachi K., Kiyonari H., Ota M., Ralston A., Yabuta N., Hirahara S., Stephenson R. O., Ogonuki N., Makita R., Kurihara H., Morin-Kensicki E. M., Nojima H., Rossant J., Nakao K., Niwa H., Sasaki H. (2009) The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 [DOI] [PubMed] [Google Scholar]
- 19.Nishioka N., Yamamoto S., Kiyonari H., Sato H., Sawada A., Ota M., Nakao K., Sasaki H. (2008) Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech. Dev. 125, 270–283 [DOI] [PubMed] [Google Scholar]
- 20.Fu V., Plouffe S. W., Guan K. L. (2017) The hippo pathway in organ development, homeostasis, and regeneration. Curr. Opin. Cell Biol. 49, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee D. H., Park J. O., Kim T. S., Kim S. K., Kim T. H., Kim M. C., Park G. S., Kim J. H., Kuninaka S., Olson E. N., Saya H., Kim S. Y., Lee H., Lim D. S. (2016) LATS-YAP/TAZ controls lineage specification by regulating TGFβ signaling and Hnf4α expression during liver development. Nat. Commun. 7, 11961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen Q., Anders R. A., Alpini G., Bai H. (2015) Yes-associated protein in the liver: regulation of hepatic development, repair, cell fate determination and tumorigenesis. Dig. Liver Dis. 47, 826–835 [DOI] [PubMed] [Google Scholar]
- 23.Miesfeld J. B., Gestri G., Clark B. S., Flinn M. A., Poole R. J., Bader J. R., Besharse J. C., Wilson S. W., Link B. A. (2015) Yap and Taz regulate retinal pigment epithelial cell fate. Development 142, 3021–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeill H., Reginensi A. (2017) Lats1/2 regulate Yap/Taz to control nephron progenitor epithelialization and inhibit myofibroblast formation. J. Am. Soc. Nephrol. 28, 852–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yimlamai D., Christodoulou C., Galli G. G., Yanger K., Pepe-Mooney B., Gurung B., Shrestha K., Cahan P., Stanger B. Z., Camargo F. D. (2014) Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R., Li Y., Hu E., Kong F., Wang J., Liu J., Shao Q., Hao Y., He D., Xiao X. (2017) S100A7 promotes lung adenocarcinoma to squamous carcinoma transdifferentiation, and its expression is differentially regulated by the Hippo-YAP pathway in lung cancer cells. Oncotarget 8, 24804–24814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St John M. A., Tao W., Fei X., Fukumoto R., Carcangiu M. L., Brownstein D. G., Parlow A. F., McGrath J., Xu T. (1999) Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat. Genet. 21, 182–186 [DOI] [PubMed] [Google Scholar]
- 28.Kawamura K., Cheng Y., Suzuki N., Deguchi M., Sato Y., Takae S., Ho C. H., Kawamura N., Tamura M., Hashimoto S., Sugishita Y., Morimoto Y., Hosoi Y., Yoshioka N., Ishizuka B., Hsueh A. J. (2013) Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 110, 17474–17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu-Chittenden Y., Huang B., Shim J. S., Chen Q., Lee S. J., Anders R. A., Liu J. O., Pan D. (2012) Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y., Feng Y., Jansson L., Sato Y., Deguchi M., Kawamura K., Hsueh A. J. (2015) Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J. 29, 2423–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., Martin J. F. (2011) Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 34.Svotelis A., Gévry N., Gaudreau L. (2009) Chromatin immunoprecipitation in mammalian cells. Methods Mol. Biol. 543, 243–251 [DOI] [PubMed] [Google Scholar]
- 35.Park G. S., Oh H., Kim M., Kim T., Johnson R. L., Irvine K. D., Lim D. S. (2016) An evolutionarily conserved negative feedback mechanism in the hippo pathway reflects functional difference between LATS1 and LATS2. Oncotarget 7, 24063–24075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan H. Y., Liu Z., Cahill N., Richards J. S. (2008) Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol. Endocrinol. 22, 2128–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan H. Y., Shimada M., Liu Z., Cahill N., Noma N., Wu Y., Gossen J., Richards J. S. (2008) Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 135, 2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sforza C., Vizzotto L., Ferrario V. F., Forabosco A. (2003) Position of follicles in normal human ovary during definitive histogenesis. Early Hum. Dev. 74, 27–35 [DOI] [PubMed] [Google Scholar]
- 39.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., Fassina A., Cordenonsi M., Piccolo S. (2014) YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 [DOI] [PubMed] [Google Scholar]
- 40.Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., Cordenonsi M., Piccolo S. (2012) Role of TAZ as mediator of Wnt signaling. Cell 151, 1443–1456 [DOI] [PubMed] [Google Scholar]
- 41.Kent J., Wheatley S. C., Andrews J. E., Sinclair A. H., Koopman P. (1996) A male-specific role for SOX9 in vertebrate sex determination. Development 122, 2813–2822 [DOI] [PubMed] [Google Scholar]
- 42.Ducy P., Karsenty G. (1995) Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15, 1858–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song S., Ajani J. A., Honjo S., Maru D. M., Chen Q., Scott A. W., Heallen T. R., Xiao L., Hofstetter W. L., Weston B., Lee J. H., Wadhwa R., Sudo K., Stroehlein J. R., Martin J. F., Hung M. C., Johnson R. L. (2014) Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 74, 4170–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui C. B., Cooper L. F., Yang X., Karsenty G., Aukhil I. (2003) Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Mol. Cell. Biol. 23, 1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dzafic E., Stimpfel M., Virant-Klun I. (2013) Plasticity of granulosa cells: on the crossroad of stemness and transdifferentiation potential. J. Assist. Reprod. Genet. 30, 1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kossowska-Tomaszczuk K., De Geyter C., De Geyter M., Martin I., Holzgreve W., Scherberich A., Zhang H. (2009) The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells 27, 210–219 [DOI] [PubMed] [Google Scholar]
- 47.Varras M., Griva T., Kalles V., Akrivis C., Paparisteidis N. (2012) Markers of stem cells in human ovarian granulosa cells: is there a clinical significance in ART? J. Ovarian Res. 5, 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattioli M., Gloria A., Turriani M., Berardinelli P., Russo V., Nardinocchi D., Curini V., Baratta M., Martignani E., Barboni B. (2012) Osteo-regenerative potential of ovarian granulosa cells: an in vitro and in vivo study. Theriogenology 77, 1425–1437 [DOI] [PubMed] [Google Scholar]
- 49.Oki Y., Ono H., Motohashi T., Sugiura N., Nobusue H., Kano K. (2012) Dedifferentiated follicular granulosa cells derived from pig ovary can transdifferentiate into osteoblasts. Biochem. J. 447, 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boerboom D., White L. D., Dalle S., Courty J., Richards J. S. (2006) Dominant-stable beta-catenin expression causes cell fate alterations and Wnt signaling antagonist expression in a murine granulosa cell tumor model. Cancer Res. 66, 1964–1973 [DOI] [PubMed] [Google Scholar]
- 51.Boerboom D., Paquet M., Hsieh M., Liu J., Jamin S. P., Behringer R. R., Sirois J., Taketo M. M., Richards J. S. (2005) Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 65, 9206–9215 [DOI] [PubMed] [Google Scholar]
- 52.Uhlenhaut N. H., Jakob S., Anlag K., Eisenberger T., Sekido R., Kress J., Treier A. C., Klugmann C., Klasen C., Holter N. I., Riethmacher D., Schütz G., Cooney A. J., Lovell-Badge R., Treier M. (2009) Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130–1142 [DOI] [PubMed] [Google Scholar]
- 53.Lindeman R. E., Gearhart M. D., Minkina A., Krentz A. D., Bardwell V. J., Zarkower D. (2015) Sexual cell-fate reprogramming in the ovary by DMRT1. Curr. Biol. 25, 764–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maatouk D. M., DiNapoli L., Alvers A., Parker K. L., Taketo M. M., Capel B. (2008) Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum. Mol. Genet. 17, 2949–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrionuevo F. J., Hurtado A., Kim G. J., Real F. M., Bakkali M., Kopp J. L., Sander M., Scherer G., Burgos M., Jiménez R. (2016) Sox9 and Sox8 protect the adult testis from male-to-female genetic reprogramming and complete degeneration. Elife 5, 15635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levasseur A., Paquet M., Boerboom D., Boyer A. (2017) Yes-associated protein and WW-containing transcription regulator 1 regulate the expression of sex-determining genes in sertoli cells, but their inactivation does not cause sex reversal. Biol. Reprod. 97, 162–175 [DOI] [PubMed] [Google Scholar]
- 57.Li Y., Zhang L., Hu Y., Chen M., Han F., Qin Y., Chen M., Cui X., Duo S., Tang F., Gao F. (2017) β-Catenin directs the transformation of testis sertoli cells to ovarian granulosa-like cells by inducing Foxl2 expression. J. Biol. Chem. 292, 17577–17586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roca H., Franceschi R. T. (2008) Analysis of transcription factor interactions in osteoblasts using competitive chromatin immunoprecipitation. Nucleic Acids Res. 36, 1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teplyuk N. M., Galindo M., Teplyuk V. I., Pratap J., Young D. W., Lapointe D., Javed A., Stein J. L., Lian J. B., Stein G. S., van Wijnen A. J. (2008) Runx2 regulates G protein-coupled signaling pathways to control growth of osteoblast progenitors. J. Biol. Chem. 283, 27585–27597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang Y., Feinberg T., Keller E. T., Li X. Y., Weiss S. J. (2016) Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat. Cell Biol. 18, 917–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heath E., Tahri D., Andermarcher E., Schofield P., Fleming S., Boulter C. A. (2008) Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev. Biol. 8, 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hindley C. J., Condurat A. L., Menon V., Thomas R., Azmitia L. M., Davis J. A., Pruszak J. (2016) The hippo pathway member YAP enhances human neural crest cell fate and migration. Sci. Rep. 6, 23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang Z., Hu J., Pan J., Wang Y., Hu G., Zhou J., Mei L., Xiong W. C. (2016) YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development 143, 2398–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poon C. L., Mitchell K. A., Kondo S., Cheng L. Y., Harvey K. F. (2016) The hippo pathway regulates neuroblasts and brain size in Drosophila melanogaster. Curr. Biol. 26, 1034–1042 [DOI] [PubMed] [Google Scholar]
- 65.Malik A. R., Liszewska E., Jaworski J. (2015) Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front. Cell. Neurosci. 9, 237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen T., You Y., Jiang H., Wang Z. Z. (2017) Epithelial-mesenchymal transition (EMT): a biological process in the development, stem cell differentiation, and tumorigenesis. J. Cell. Physiol. 232, 3261–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., Pei X. H., Zhao S., Xiong Y., Guan K. L. (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 28, 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H., von Gise A., Liu Q., Hu T., Tian X., He L., Pu W., Huang X., He L., Cai C. L., Camargo F. D., Pu W. T., Zhou B. (2014) Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J. Biol. Chem. 289, 18681–18692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalluri R., Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.