Abstract
High-frequency burstlike electrical conditioning stimulation (HFS) applied to human skin induces an increase in mechanical pinprick sensitivity of the surrounding unconditioned skin (a phenomenon known as secondary hyperalgesia). The present study assessed the effect of frequency of conditioning stimulation on the development of this increased pinprick sensitivity in humans. In a first experiment, we compared the increase in pinprick sensitivity induced by HFS, using monophasic non-charge-compensated pulses and biphasic charge-compensated pulses. High-frequency stimulation, traditionally delivered with non-charge-compensated square-wave pulses, may induce a cumulative depolarization of primary afferents and/or changes in pH at the electrode-tissue interface due to the accumulation of a net residue charge after each pulse. Both could contribute to the development of the increased pinprick sensitivity in a frequency-dependent fashion. We found no significant difference in the increase in pinprick sensitivity between HFS delivered with charge-compensated and non-charge-compensated pulses, indicating that the possible contribution of charge accumulation when non-charge-compensated pulses are used is negligible. In a second experiment, we assessed the effect of different frequencies of conditioning stimulation (5, 20, 42, and 100 Hz) using charge-compensated pulses on the development of increased pinprick sensitivity. The maximal increase in pinprick sensitivity was observed at intermediate frequencies of stimulation (20 and 42 Hz). It is hypothesized that the stronger increase in pinprick sensitivity at intermediate frequencies may be related to the stronger release of substance P and/or neurokinin-1 receptor activation expressed at lamina I neurons after C-fiber stimulation.
NEW & NOTEWORTHY Burstlike electrical conditioning stimulation applied to human skin induces an increase in pinprick sensitivity in the surrounding unconditioned skin (a phenomenon referred to as secondary hyperalgesia). Here we show that the development of the increase in pinprick sensitivity is dependent on the frequency of the burstlike electrical conditioning stimulation.
Keywords: frequency, high-frequency stimulation, pinprick, secondary hyperalgesia
INTRODUCTION
Long-term potentiation (LTP) refers to a long-lasting increase in synaptic efficacy and was discovered after repetitive stimulation in the rabbit hippocampus (Bliss and Gardner-Medwin 1973; Bliss and Lømo 1973; Lømo 2003). LTP is thought to be a crucial mechanism involved in memory formation (Bliss and Collingridge 1993; Neves et al. 2008). Animal studies have shown that LTP can also be induced in spinal nociceptive pathways. Indeed, it has been shown that high-frequency burstlike stimulation (HFS; several bursts of 100 Hz for 1 s) of primary C-fiber nociceptors triggers LTP between the peripheral C-fiber terminals and spinal lamina I neurons projecting to the parabrachial area of the brain stem (Ikeda et al. 2003, 2006). Moreover, Kronschläger et al. (2016) showed that HFS also activates glial cells in the spinal cord that, via the release of d-serine and tumor necrosis factor, trigger LTP at remote or nearby C-fiber synapses. LTP at synapses that were active during conditioning stimulation (homosynaptic LTP) may contribute, besides peripheral sensitization, to primary hyperalgesia, i.e., the increase in pain at the site of tissue injury or inflammation (Sandkühler and Gruber-Schoffnegger 2012). LTP at remote synapses (heterosynaptic LTP) could contribute to the phenomenon of secondary hyperalgesia, i.e., the increase in pain sensitivity that develops surrounding the site of tissue injury (Kronschläger et al. 2016; Ruscheweyh et al. 2011).
In humans, HFS (5 trains of 100 Hz for 1 s, repeated at 10-s intervals) delivered to the skin induces a pronounced and long-lasting increase in mechanical pinprick sensitivity in the surrounding skin, a phenomenon reminiscent of secondary hyperalgesia (Henrich et al. 2015; Klein et al. 2004; van den Broeke et al. 2016; Vo and Drummond 2013; Xia et al. 2016).
We also showed that after HFS the perception elicited by small-spot laser stimuli selectively activating C-fiber nociceptors is enhanced when the stimuli are delivered inside the surrounding area of increased pinprick sensitivity, although the effect of HFS on these laser stimuli was less pronounced than the effect on pinprick stimuli (Lenoir et al. 2018). The effect of HFS on the perception elicited by the C-fiber laser stimuli could be a perceptual correlate of the “gliogenic” heterosynaptic LTP at C-fiber synapses identified by Kronschläger et al. (2016) in animals. However, a peripheral origin cannot presently be excluded.
Previous studies using intradermal capsaicin injection to induce increased pinprick sensitivity surrounding the site of injection in humans have shown that the increase in pinprick sensitivity is mediated by A-fiber nociceptors rather than C fibers (Ziegler et al. 1999). Moreover, by recording the activity of nociceptive neurons in the primate spinal cord before and after intradermal capsaicin injection, Simone et al. (1991) showed that after the injection both high-threshold (HT) neurons in lamina I and wide-dynamic-range (WDR) neurons in lamina V respond more strongly to mechanical pinprick stimuli delivered to the skin surrounding the injection site. The same group also recorded the activity of peripheral A-fiber and C-fiber nociceptors, but their activity was unchanged (Baumann et al. 1991), confirming that the increase in responsiveness of spinal neurons results from a facilitation at spinal level. Torsney (2011) found that inflammation of the hind paw of rats by complete Freund’s adjuvant increases the incidence and magnitude of monosynaptic A-fiber input to lamina I neurons expressing the neurokinin-1 (NK1) receptor. It was hypothesized that this novel monosynaptic A-fiber input results from normally “silent” synapses and that this may contribute to secondary hyperalgesia (Torsney 2011). It is, however, presently not known whether spinal LTP also affects A-fiber-mediated synaptic transmission (Ruscheweyh et al. 2011).
Henrich et al. (2015) showed that when HFS is delivered to skin pretreated with capsaicin to induce a denervation of transient receptor potential vanilloid (TRPV)1-expressing nociceptors HFS does not induce any longlasting increase in pinprick sensitivity in the surrounding skin. They also showed that both A- and C-fiber nociceptors contribute to the induction of increased pinprick sensitivity, but the contribution of C-fiber input is greater than that of A-fibers (Henrich et al. 2015). Taken together, these results suggest that mainly TRPV1-expressing C-fiber nociceptors are involved in the HFS-induced enhancement of pinprick sensitivity (Henrich et al. 2015).
It is thought that the activation of mechano-insensitive “silent” C-fiber nociceptors is crucial for the induction of secondary mechanical hyperalgesia (Sauerstein et al. 2018; Schmelz et al. 2000). However, in pig skin this subclass of nociceptors shows conduction failure at high frequencies of stimulation (Obreja et al. 2013; Werland et al. 2015), which raises the question of the extent to which this subclass of C-fibers contributes to the induction of secondary mechanical hyperalgesia by HFS. However, in rats some C-fiber nociceptors are able to follow HFS (Adelson et al. 2009).
Not much is known about the effect of frequency of the conditioning stimulation on the development of the increased pinprick sensitivity in humans. Xia et al. (2016) investigated the effect of three frequencies of electrical conditioning stimulation (10, 100, and 200 Hz) on the averaged magnitude of the increase in pinprick sensitivity in the surrounding skin. Although the magnitude of the increase in pinprick sensitivity was not the same between the different frequencies (100 Hz > 200 Hz > 10 Hz), no statistically significant differences were observed. In that study, the authors matched the 10-Hz and 100-Hz frequencies regarding the total number of stimuli, pulse duration, and total duration of the protocol. As a consequence, the pattern of stimulation was not the same, which makes it difficult to compare the two conditions. Indeed, whereas the 100-Hz condition consisted of five trains of 100 Hz that lasted for 1 s and were repeated in a 10-s interval, the 10-Hz condition consisted of continuous stimulation. Another previous study comparing 20-Hz continuous stimulation versus 20-Hz stimulation for 1 s repeated with a 2-s intertrain interval found that continuous stimulation induces hypoalgesia to pinprick stimulation, whereas burst stimulation induces hyperalgesia to pinprick stimulation (De Col and Maihöfner 2008).
Furthermore, in the study by Xia et al. (2016), and other studies exploring how frequency influences the aftereffects of the conditioning stimulation, they used square-wave electrical pulses. Because square-wave electrical pulses are not charge compensated, a net residue charge may accumulate after each pulse. This accumulation can be expected to be stronger when the frequency of pulse delivery is high, leading to a stronger cumulative depolarization of the membrane potential of afferent fibers (Grover et al. 2009) and/or tissue damage (Piallat et al. 2009) or inflammation related to changes in pH at the electrode-tissue interface.
Therefore, the present study had two aims. The first aim was to assess whether the increase in pinprick sensitivity induced by HFS is dependent on cumulative depolarization of the membrane potential and/or inflammation related to changes in pH at the electrode-tissue interface. To test this, we compared the increase in pinprick sensitivity induced by HFS delivered with non-charge-compensated versus charge-compensated electrical pulses (experiment 1). The second aim was to explore whether the development of the increase in pinprick sensitivity depends on the frequency of the conditioning stimulation (experiment 2). If indeed mechano-insensitive “silent” C-fiber nociceptors play a crucial role, one would expect a stronger increase in pinprick sensitivity induced by low frequencies of stimulation compared with high frequencies of stimulation. In this second experiment, four frequencies were tested (5, 20, 42, and 100 Hz) with charge-compensated electrical pulses, keeping constant both the total number of pulses and the stimulation pattern (1-s trains separated by a 10-s intertrain interval).
MATERIALS AND METHODS
Participants
Fifteen healthy volunteers took part in experiment 1 [7 men and 8 women; aged 21–27 yr, 23.5 ± 1.6 yr (mean ± SD)]. In this experiment, participants took part in two experimental sessions separated by at least 1 wk, during which they were exposed to either charge-compensated 100-Hz HFS or non-charge-compensated 100-Hz HFS. The order of the two sessions was counterbalanced across participants.
Sixty participants took part in experiment 2 (31 men and 29 women; aged 18–40 yr, 23.4 ± 4.3 yr), 15 participants per condition (5, 20, 42, and 100 Hz). For the 100-Hz group, this included the data of the seven participants of experiment 1 who had received 100-Hz charge-compensated HFS in the first experimental session. All participants were naive regarding HFS.
The experiments were conducted according to the Declaration of Helsinki (except for preregistration of the trial). Approval for the experiments was obtained from the local Ethical Committee (comité d'éthique hospitalo-facultaire des Cliniques universitaires Saint-Luc-UCLouvain) of the Université catholique de Louvain (UCLouvain) (B403201316436). All participants gave written informed consent and received financial compensation for their participation.
Experimental Design
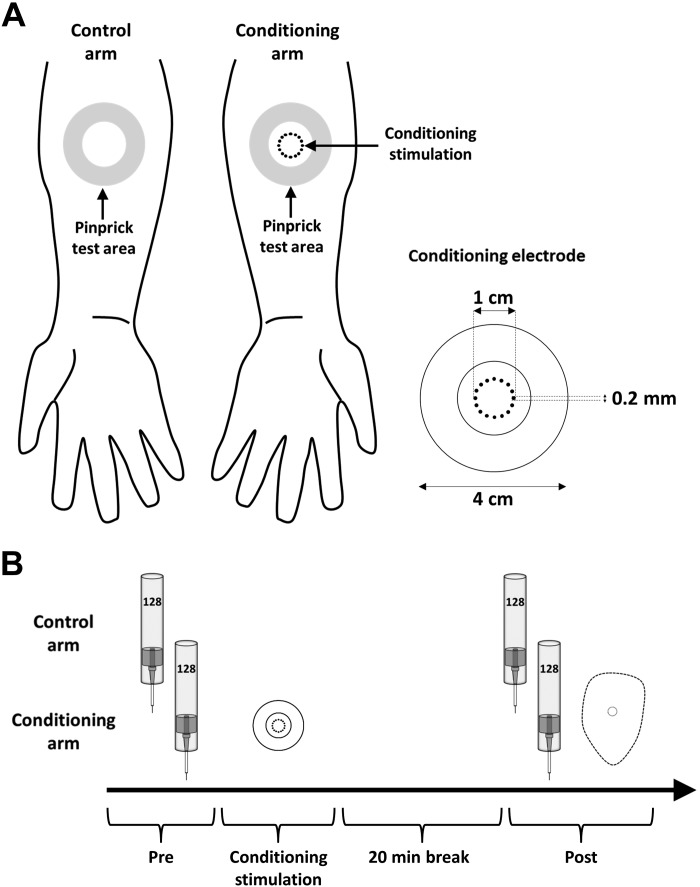
In both experiments, the electrical conditioning stimulation was applied to the dominant or nondominant volar forearm, counterbalanced across participants (10 cm distal to the cubital fossa) (Fig. 1). Handedness was assessed with the Flinders Handedness Survey (Nicholls et al. 2013). Pinprick sensitivity of the skin was assessed by applying mechanical pinprick stimuli (128 mN) before applying the conditioning stimulation (“Pre”) and 20 min after the end of the conditioning stimulation (“Post”) to the skin surrounding the site where the conditioning stimulation was delivered (“pinprick test area”) and to the corresponding skin area of the contralateral arm serving as control. In experiment 1, we compared in a crossover design the increase in pinprick sensitivity induced by 100-Hz HFS delivered with either biphasic charge-compensated pulses or monophasic non-charge-compensated pulses (Fig. 2). In experiment 2, we compared in a between-subject design the change in pinprick sensitivity induced by 100-Hz HFS to the change in pinprick sensitivity induced by 5-, 20-, and 42-Hz conditioning stimulation. In this second experiment, all stimuli were delivered with biphasic charge-compensated pulses.
Fig. 1.
Experimental design. A, left: conditioning stimulation is applied to the dominant or nondominant volar forearm. Pinprick stimulation (128 mN) was applied to the skin surrounding the area onto which conditioning stimulation was applied (“pinprick test area”) as well as to the same skin area on the contralateral control arm. Right: characteristics of the conditioning electrode. B: timeline of the experiment. The perceived intensity elicited by the pinprick stimulation was assessed at 2 different time points: before conditioning stimulation (“Pre”) and 20 min after application of conditioning stimulation (“Post”). At the end of the experiment the area of increased pinprick sensitivity at the conditioning arm was mapped.
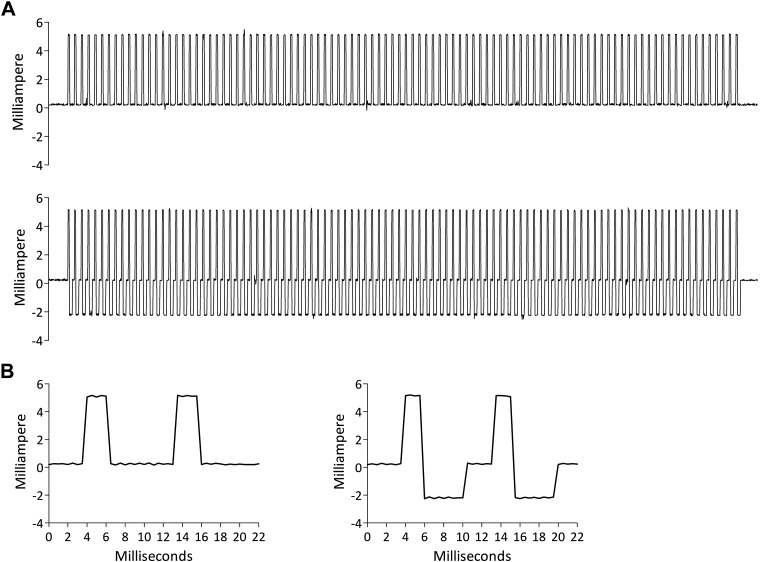
Fig. 2.
A: example of a train of 100 Hz (1 s) delivered with a non-charge-compensated pulse (top) and a charge-compensated pulse (bottom). Shown is the actual current delivered to the skin (stimulation intensity: 5 mA). B: first 2 stimuli of each train in A. Left: non-charge-compensated pulses. Right: charge-compensated pulses.
Conditioning Stimulation
All stimuli were delivered to the forearm skin with a constant-current electrical stimulator (Digitimer DS5, Welwyn Garden City, UK) and a specifically designed electrode built at the Center for Sensory-Motor Interaction (Aalborg University, Aalborg, Denmark). The electrode consists of 16 blunt stainless steel pins (diameter: 0.2 mm) protruding 1 mm from the base. The pins are placed in a 10-mm-diameter circle and serve as cathode. A stainless steel anode electrode is concentrically located around the steel pins (inner diameter: 22 mm; outer diameter: 40 mm).
Monophasic non-charge-compensated electrical pulses were square-wave pulses with a 2-ms pulse width (Fig. 2B). Biphasic charge-compensated electrical pulses consisted of the same 2-ms square-wave pulse followed, after a 0.1-ms delay, by a 4-ms compensation pulse of opposite polarity with half the intensity of the first pulse (Fig. 2B).
In all conditions, intensity of the conditioning stimulation was individually adjusted to 20× the detection threshold to a single non-charge-compensated monophasic pulse (pulse width: 2 ms). The detection threshold was determined after the Pre measurements with a staircase procedure.
A total of 500 electrical pulses were delivered as 1-s trains separated by a stimulation-free interval lasting 9 s. For 100-Hz HFS, five trains were delivered, each including 100 pulses (total duration: 50 s). For 5-Hz stimulation, a total of 100 trains were delivered, each including 5 pulses (total duration: ≈17 min). For 20-Hz stimulation, 25 trains were delivered, each including 20 pulses (total duration: ≈4 min). For 42-Hz stimulation, 12 trains were delivered, 8 including 42 pulses and 4 including 41 pulses (total duration: ≈2 min). The 42-Hz stimulation was chosen instead of 40 Hz (the double of 20) because it is able to deliver the same total number of stimuli as the 5-Hz, 20-Hz, and 100-Hz stimulations.
The electrical pulses were triggered by a National Instruments digital-analog interface (NI; National Instruments, Austin, TX) controlled by custom MATLAB code (MATLAB 2014B; MathWorks).
Quantifying Changes in Perceived Intensity of Mechanical Pinprick Stimuli
To assess changes in pinprick sensitivity, a calibrated pinprick stimulator exerting a normal force of 128 mN with a 0.25-mm probe (MRC Systems, Heidelberg, Germany) was applied perpendicular to the skin. Before application of the conditioning stimulation and 20 min after application of the conditioning stimulation a total of three pinprick stimuli were applied inside the pinprick test area of the conditioned arm and the contralateral control arm. The target of each pinprick stimulus was displaced after each stimulus. Participants were asked to report the intensity of perception elicited by the pinprick stimulation on a numerical rating scale ranging from 0 (no perception) to 100 (maximal pain), with 50 representing the transition from nonpainful to painful domains of sensation. For the statistical analysis, the mean of the three pinprick ratings was calculated for each arm and time point.
Mapping Area of Increased Pinprick Sensitivity
The same pinprick stimulator was used to map the area of increased mechanical pinprick sensitivity after conditioning stimulation. Mechanical pinprick stimuli were applied to the skin along eight axes, each separated by an angle of 45°. Along each axis, testing started far outside the skin showing increased pinprick sensitivity and moved toward the center of the conditioning site in steps of 1 cm. Participants were instructed to indicate the point at which the pinprick perception changed. This point was then indicated on the skin with a marker. Then, the distance between each mark and the center of the conditioning stimulation was measured. Finally, the area was drawn on millimeter paper, and the surface (cm2) was calculated with the open-source platform Fiji (Schindelin et al. 2012).
Statistical Analysis
Statistical analyses were performed with SPSS Statistics 24 (IBM, Armonk, NY). In experiment 1, the changes in perceived pinprick intensity induced by non-charge-compensated monophasic pulses and charge-compensated biphasic pulses were compared with a repeated-measures ANOVA with three within-subject factors: “time” (Pre vs. Post), “arm” (conditioned vs. control), and “condition” (charge compensated vs. non-charge compensated). Post hoc paired t-tests were performed comparing the Post minus Pre change in perception intensity at the conditioned arm versus the control arm. To compare the size of the area of increased pinprick sensitivity after charge-compensated versus non-charge-compensated HFS, we performed a paired t-test on the individual area sizes (cm2).
In experiment 2, the change in intensity of pinprick perception after conditioning stimulation using four frequencies of stimulation was compared with a mixed ANOVA with two within-subject factors, “time” (Pre vs. Post) and “arm” (conditioned vs. control), and one between-subject factor, “condition” (5, 20, 42, and 100 Hz). Tukey post hoc tests were performed comparing the Post minus Pre change in pinprick intensity ratings at the conditioned arm versus the control arm. The size of the area of increased pinprick sensitivity was compared across the four frequencies of stimulation (5, 20, 42, and 100 Hz) with a one way-ANOVA. A Tukey post hoc test was performed to identify which comparisons were significantly different.
Finally, to test whether the electrical detection thresholds to a monophasic non-charge-compensated pulse differed in the two experimental sessions of experiment 1, the individual detection thresholds were compared by paired t-test. To test whether in experiment 2 the detection thresholds differed between the four different groups (5, 20, 42, and 100 Hz), the individual detection thresholds were compared with a one-way ANOVA. In all tests, the level of significance was set at P < 0.05.
RESULTS
Detection Thresholds
The electrical detection thresholds to a single monophasic non-charge-compensated pulse in experiment 1 were 0.29 ± 0.13 mA (mean ± SD) for the non-charge-compensated condition and 0.32 ± 0.11 mA for the charge-compensated condition. The electrical detection thresholds in experiment 2 were 0.25 ± 0.09 (5 Hz), 0.27 ± 0.10 (20 Hz), 0.29 ± 0.09 (42 Hz), and 0.29 ± 0.12 (100 Hz). No significant difference in electrical detection thresholds was observed between the charge-compensated and non-charge-compensated conditions of experiment 1 and between the four groups in experiment 2.
Experiment 1
Intensity of pinprick perception.
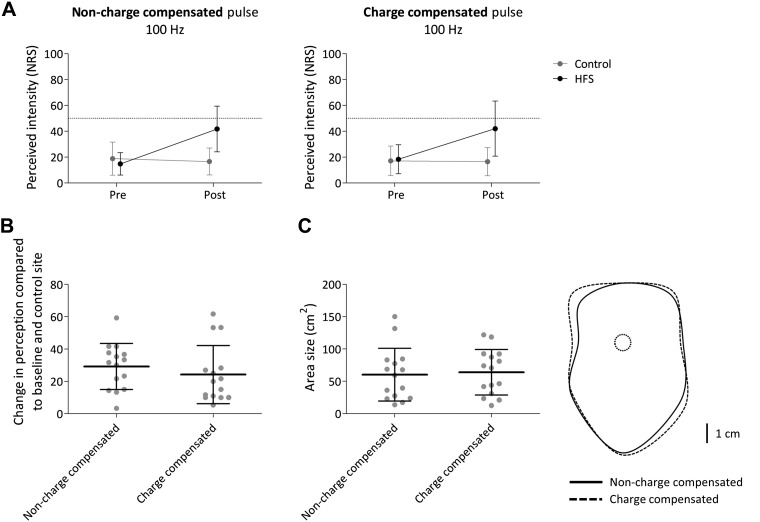
The means and SDs of the intensity of perception elicited by pinprick stimuli delivered before and after HFS at both arms (control vs. conditioned) in both conditions (charge-compensated vs. non-charge-compensated pulses) are shown in Fig. 3A. The repeated-measures ANOVA revealed a significant time × arm interaction [F(1,14) = 54.684, P < 0.001, η2 = 0.796]. This means that after HFS the intensity of perception elicited by pinprick stimulation of the HFS arm was higher compared with pinprick stimulation of the control arm across the two conditions (charge-compensated and non-charge-compensated stimulation) (Fig. 3A). No significant time × arm × condition interaction was observed [F(1,14) = 1.392, P = 0.258, η2 = 0.090], suggesting that there was no difference in the enhancement of pinprick sensitivity after HFS delivered with charge-compensated and non-charge-compensated pulses (Fig. 3B).
Fig. 3.
A: intensity of perception elicited by the mechanical pinprick stimulation (128 mN) before and 20 min after application of high-frequency burstlike stimulation (HFS) using a monophasic non-charge-compensated pulse (left) and a biphasic charge-compensated pulse (right). Shown are the group-level average and standard deviation (SD) of the numerical rating scale scores (NRS). B: group-level average and SD increase in NRS compared with baseline and control site. C, left: group-level average and SD area size of the increase in pinprick sensitivity. Right: group-level average areas of increased pinprick sensitivity.
Area size.
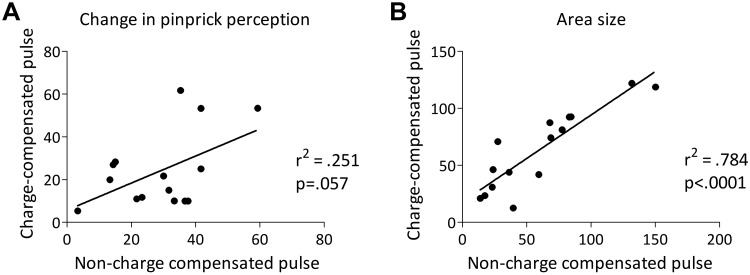
The means and SDs of the area of increased pinprick sensitivity after charge-compensated and non-charge-compensated HFS are shown in Fig. 3C. The paired t-test comparing area sizes revealed no significant difference between charge-compensated and non-charge-compensated pulses [t(14) = −0.738, P = 0.472]. Figure 4 shows scatterplots of the individual changes in pinprick perception and area size after HFS delivered with a charge-compensated pulse versus a non-charge-compensated pulse.
Fig. 4.
A: scatterplot (and linear regression line) showing the individual changes in pinprick ratings after high-frequency burstlike stimulation (HFS) (compared with baseline and control site) delivered with charge-compensated pulses (y-axis) and non-charge-compensated pulses (x-axis). B: scatterplot (and linear regression line) showing the individual area sizes of increased pinprick sensitivity after HFS delivered with charge-compensated pulses (y-axis) and non-charge-compensated pulses (x-axis).
Experiment 2
Intensity of perception.
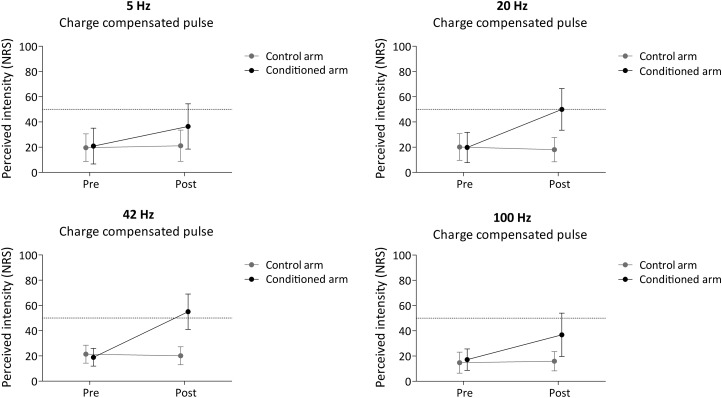
The means and SDs of the intensity of pinprick perception before and after conditioning stimulation at both arms (conditioned vs. control) in all four groups (5, 20, 42, and 100 Hz) are shown in Fig. 5. The mixed ANOVA revealed a significant time × arm interaction [F(1,56) = 179.621, P < 0.001, η2 = 0.762], compatible with an increase in pinprick perception at the conditioned forearm in all four groups (5, 20, 42, and 100 Hz). Most importantly, there was a significant time × arm × condition interaction [F(3,56) = 8.493, P < 0.001, η2 = 0.313], indicating that the strength of the increase of pinprick perception at the conditioned arm differed across the four frequencies of stimulation.
Fig. 5.
Intensity of perception elicited by the mechanical pinprick stimulation (128 mN) before and 20 min after application of high-frequency burstlike stimulation (HFS) using a biphasic charge-compensated pulse for all frequencies of conditioning stimulation (5, 20, 42, and 100 Hz). Shown are the group-level average and standard deviation of the numerical rating scale scores (NRS).
To assess whether the increase in pinprick sensitivity was significant in all four groups, we then performed, for each group of participants, separate repeated-measures ANOVAs with the factors of time and arm. For all four frequencies of stimulation, there was a significant time × arm interaction [5 Hz: F(1,14) = 26.846, P < 0.001, η2 = 0.657; 20 Hz: F(1,14) = 58.031, P < 0.001, η2 = 0.806; 42 Hz: F(1,14) = 86.701, P < 0.001, η2 = 0.861; 100 Hz: F(1,14) = 20.459, P < 0.001, η2 = 0.594].
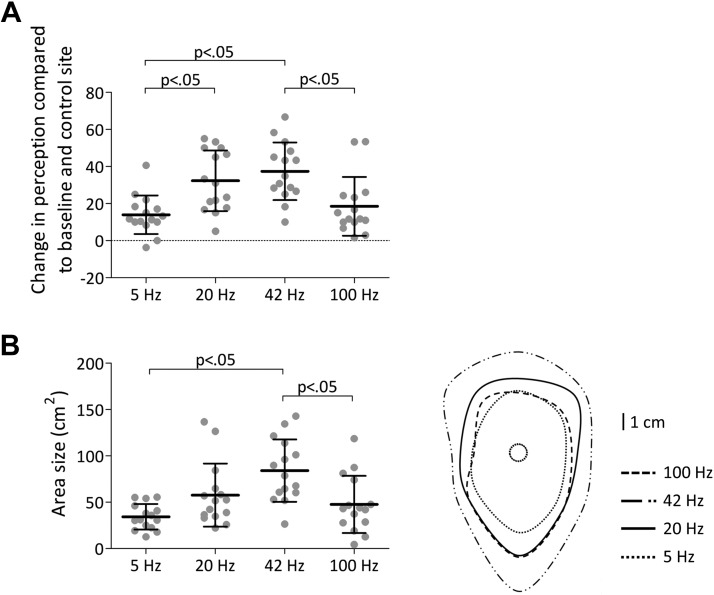
Tukey post hoc tests performed on the Post minus Pre change in pinprick perception at the conditioned arm versus the control arm revealed a significant difference between 5- and 20-Hz stimulation (P = 0.007), between 5- and 42-Hz stimulation (P < 0.001), and between 42- and 100-Hz stimulation (P = 0.005) (Fig. 6A).
Fig. 6.
A: group-level average and standard deviation (SD) increase in numerical rating scale score compared with baseline and control site. B, left: group-level average and SD area size of the increase in pinprick sensitivity. P < 0.05 refers to the significant comparisons of the post hoc Tukey test. Right: group-level average areas of increased pinprick sensitivity.
Area size.
The means and SDs of the area of increased pinprick sensitivity after 5-, 20-, 42-, and 100-Hz conditioning stimulation are shown in Fig. 6B. One-way ANOVA revealed a statistically significant difference between the different frequencies [F(3,59) = 7.781, P < 0.001]. Tukey post hoc tests revealed a significant difference between 5- and 42-Hz stimulation (P < 0.001) and between 42- and 100-Hz stimulation (P = 0.006) (Fig. 6B).
DISCUSSION
The present study yields two important findings. First, there is no significant difference in the intensity and area size of the increase in pinprick sensitivity induced by 100-Hz HFS delivered with charge-compensated and non-charge-compensated pulses. This result indicates that HFS is able to induce increased pinprick sensitivity even when the conditioning pulses are charge compensated and that the possible contribution of cumulative depolarization of sensory afferents and/or tissue lesion or inflammation induced by charge accumulation when non-charge-compensated pulses are used is negligible.
Second, we show that the increase in pinprick sensitivity, which is thought to result from spinal heterosynaptic facilitation, is dependent on the frequency of conditioning stimulation. Indeed, with a constant number of electrical pulses delivered with the same pattern of stimulation (1-s trains delivered every 10 s), intermediate frequencies of stimulation (20 and 42 Hz) induce a stronger increase in pinprick sensitivity compared with both high-frequency stimulation (100 Hz) and low-frequency stimulation (5 Hz).
At present, one can only speculate about the possible mechanism(s) underlying the frequency dependence of HFS-induced increase in pinprick sensitivity. One possibility could be that the frequency-dependent increase in pinprick sensitivity is related to spinal NK1 activation through the release of substance P following primary afferent peptidergic A- and C-fiber nociceptor stimulation. Both the release of substance P and the activation of the NK1 receptor are frequency dependent (Adelson et al. 2009; Go and Yaksh 1987). Indeed, Go and Yaksh (1987) showed in cats that the release of substance P after sciatic nerve stimulation at 2, 5, 10, 20, 50, and 200 Hz was largest at 20 and 50 Hz and then decreased. Furthermore, Adelson et al. (2009) showed in rats that NK1 receptor activation was maximal when C fibers were stimulated at frequencies between 30 and 100 Hz. Moreover, substance P can diffuse at a considerable distance from its site of release (Liu et al. 1994) and may be able to activate extrasynaptic NK1 receptors (Klein et al. 2008). Moreover, animal studies have shown that spinal lamina I neurons expressing the NK1 receptor play a pivotal role in central sensitization and mechanical hyperalgesia (Abbadie et al. 1997; Khasabov et al. 2002; Mantyh et al. 1997; Nichols et al. 1999).
That high-frequency stimulation induces an increase in pinprick sensitivity similar to low-frequency stimulation is somewhat surprising, as the aforementioned studies have shown that high-frequency stimulation results in a greater release of substance P and more NK1 activation than low-frequency stimulation (Adelson et al. 2009; Go and Yaksh 1987). One possibility is that HFS triggers LTP at GABAergic synapses of spinal lamina I neurons that receive monosynaptic A- or C-fiber input (Fenselau et al. 2011), which may influence the net output (less facilitation) of these lamina I neurons. Second, HFS may recruit more strongly descending inhibitory pathways that may interact with the development of increased pinprick sensitivity. In animals, intense nociceptive stimulation recruits diffuse inhibitory noxious controls (DNICs), which can inhibit the activity of WDR neurons of the dorsal horn (LeBars 2002). Simone et al. (1991) showed in primates that both HT neurons (in the superficial laminae) and WDR neurons (in deeper lamina) show increased responses to pinprick stimulation when these pinprick stimuli are applied after intradermal capsaicin injection in the surrounding skin, suggesting that WDR neurons also contribute to the increase in pinprick sensitivity, at least after capsaicin. That a DNIC-like mechanism can interfere with the development of increased pinprick sensitivity has been shown recently by Xia et al. (2017). In that study they showed that 10-Hz conditioning stimulation of the forearm skin delivered just after the application of conditioned pain modulation to the foot, which is believed to recruit a DNIC-like mechanism (Bannister and Dickenson 2017; Yarnitsky 2010), induces a smaller increase in pinprick sensitivity compared with a control condition not preceded by conditioned pain modulation.
In summary, our results show that the induction of increased pinprick sensitivity by repeated burstlike electrical stimulation of cutaneous nociceptors is not significantly dependent on charge accumulation within the stimulated tissues, and that the induced increased pinprick sensitivity is significantly dependent on the frequency of the burst stimulation, being maximal at intermediate frequencies of stimulation.
GRANTS
E. N. van den Broeke is supported by the Fonds de Recherche Clinique provided by the Université catholique de Louvain. A. Mouraux, A. Stouffs, and L. Lebrun are supported by a European Research Council “Starting Grant” (PROBING PAIN 336130). S. G. A. van Neerven is supported by the MoveIN-Louvain fellowship provided by the Université catholique de Louvain.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.N.v.d.B., S.G.v.N., and A.M. conceived and designed research; S.G., J.B., A.S., and L.L. performed experiments; E.N.v.d.B., S.G., J.B., A.S., and S.G.v.N. analyzed data; E.N.v.d.B., S.G., J.B., A.S., L.L., S.G.v.N., and A.M. interpreted results of experiments; E.N.v.d.B. prepared figures; E.N.v.d.B., S.G., J.B., A.S., L.L., S.G.v.N., and A.M. drafted manuscript; E.N.v.d.B., S.G.v.N., and A.M. edited and revised manuscript; E.N.v.d.B., S.G., J.B., A.S., L.L., S.G.v.N., and A.M. approved final version of manuscript.
REFERENCES
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci 17: 8049–8060, 1997. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson D, Lao L, Zhang G, Kim W, Marvizón JC. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increase with the firing frequency of C-fibers. Neuroscience 161: 538–553, 2009. doi: 10.1016/j.neuroscience.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister K, Dickenson AH. The plasticity of descending controls in pain: translational probing. J Physiol 595: 4159–4166, 2017. doi: 10.1113/JP274165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 66: 212–227, 1991. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetized rabbit following stimulation of the perforant path. J Physiol 232: 357–374, 1973. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232: 331–356, 1973. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Col R, Maihöfner C. Centrally mediated sensory decline induced by differential C-fiber stimulation. Pain 138: 556–564, 2008. doi: 10.1016/j.pain.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Fenselau H, Heinke B, Sandkühler J. Heterosynaptic long-term potentiation at GABAergic synapses of spinal lamina I neurons. J Neurosci 31: 17383–17391, 2011. doi: 10.1523/JNEUROSCI.3076-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go VL, Yaksh TL. Release of substance P from the cat spinal cord. J Physiol 391: 141–167, 1987. doi: 10.1113/jphysiol.1987.sp016731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Kim E, Cooke JD, Holmes WR. LTP in hippocampal area CA1 is induced by burst stimulation over a broad frequency range centered around delta. Learn Mem 16: 69–81, 2009. doi: 10.1101/lm.1179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich F, Magerl W, Klein T, Greffrath W, Treede RD. Capsaicin-sensitive C- and A-fibre nociceptors control long-term potentiation-like pain amplification in humans. Brain 138: 2505–2520, 2015. doi: 10.1093/brain/awv108. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkühler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299: 1237–1240, 2003. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jäger T, Sandkühler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 312: 1659–1662, 2006. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci 22: 9086–9098, 2002. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Magerl W, Hopf HC, Sandkühler J, Treede RD. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci 24: 964–971, 2004. doi: 10.1523/JNEUROSCI.1222-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Stahn S, Magerl W, Treede RD. The role of heterosynaptic facilitation in long-term potentiation (LTP) of human pain sensation. Pain 139: 507–519, 2008. doi: 10.1016/j.pain.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Kronschläger MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkühler J. Gliogenic LTP spreads widely in nociceptive pathways. Science 354: 1144–1148, 2016. doi: 10.1126/science.aah5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev 40: 29–44, 2002. doi: 10.1016/S0165-0173(02)00186-8. [DOI] [PubMed] [Google Scholar]
- Lenoir C, Plaghki L, Mouraux A, van den Broeke EN. Quickly responding C-fibre nociceptors contribute to heat hypersensitivity in the area of secondary hyperalgesia. J Physiol 596: 4443–4455, 2018. doi: 10.1113/JP275977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Brown JL, Jasmin L, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Synaptic relationship between substance P and the substance P receptor: light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc Natl Acad Sci USA 91: 1009–1013, 1994. doi: 10.1073/pnas.91.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T. The discovery of long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358: 617–620, 2003. doi: 10.1098/rstb.2002.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278: 275–279, 1997. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Thomas NA, Loetscher T, Grimshaw GM. The Flinders Handedness survey (FLANDERS): a brief measure of skilled hand preference. Cortex 49: 2914–2926, 2013. doi: 10.1016/j.cortex.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science 286: 1558–1561, 1999. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Obreja O, Werland F, Hirth M, Turnquist B, Rukwied R, Ringkamp M, Schmelz M. Maximum following frequency seperates classes of cutaneous unmyelinated nociceptors in the pig. EFIC Meeting Florence, Italy, October 9–12, 2013. [DOI] [PubMed] [Google Scholar]
- Piallat B, Chabardès S, Devergnas A, Torres N, Allain M, Barrat E, Benabid AL. Monophasic but not biphasic pulses induce brain tissue damage during monopolar high-frequency deep brain stimulation. Neurosurgery 64: 156–163, 2009. doi: 10.1227/01.NEU.0000336331.88559.CF. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkühler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain 7: 20, 2011. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol 12: 18–27, 2012. doi: 10.1016/j.coph.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerstein K, Liebelt J, Namer B, Schmidt R, Rukwied R, Schmelz M. Low-frequency stimulation of silent nociceptors induces secondary mechanical hyperalgesia in human skin. Neuroscience 387: 4–12, 2018. doi: 10.1016/j.neuroscience.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmid R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 123: 560–571, 2000. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol 66: 228–246, 1991. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Torsney C. Inflammatory pain unmasks heterosynaptic facilitation in lamina I neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 31: 5158–5168, 2011. doi: 10.1523/JNEUROSCI.6241-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broeke EN, Lenoir C, Mouraux A. Secondary hyperalgesia is mediated by heat-insensitive A-fibre nociceptors. J Physiol 594: 6767–6776, 2016. doi: 10.1113/JP272599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo L, Drummond PD. High frequency electrical stimulation concurrently induces central sensitization and ipsilateral inhibitory pain modulation. Eur J Pain 17: 357–368, 2013. doi: 10.1002/j.1532-2149.2012.00208.x. [DOI] [PubMed] [Google Scholar]
- Werland F, Hirth M, Turnquist B, Rukwied R, Ringkamp M, Schmelz M, Obreja O. Peak firing frequency seperates classes of C-nociceptors in pig skin. NeuPSIG, 5th International Congress on Neuropathic Pain Nice, France, May 14–17, 2015. [Google Scholar]
- Xia W, Mørch CD, Andersen OK. Exploration of the conditioning electrical stimulation frequencies for induction of long-term potentiation-like pain amplification in humans. Exp Brain Res 234: 2479–2489, 2016. doi: 10.1007/s00221-016-4653-1. [DOI] [PubMed] [Google Scholar]
- Xia W, Mørch CD, Matre D, Andersen OK. Exploration of conditioned pain modulation effect on long-term potentiation-like pain amplification in humans. Eur J Pain 21: 645–657, 2017. doi: 10.1002/ejp.968. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 23: 611–615, 2010. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain 122: 2245–2257, 1999. doi: 10.1093/brain/122.12.2245. [DOI] [PubMed] [Google Scholar]