Abstract
Background
The adjuvanted recombinant zoster vaccine (RZV) has demonstrated >90% efficacy against herpes zoster in adults ≥50 years of age and 68% efficacy in autologous hematopoietic stem cell transplant recipients ≥18 years of age. We report the immunogenicity and safety of RZV administered to patients with solid tumors (STs) before or at the start of a chemotherapy cycle.
Method
In this phase 2/3 observer‐blind, multicenter study (NCT01798056), patients with STs who were ≥18 years of age were randomized (1:1) to receive 2 doses of RZV or placebo 1‐2 months apart and stratified (4:1) according to the timing of the first dose with respect to the start of a chemotherapy cycle (first vaccination 8‐30 days before the start or at the start [±1 day] of a chemotherapy cycle). Anti‐glycoprotein E (gE) antibody concentrations, gE‐specific CD4+ T cell frequencies, and vaccine response rates (VRRs) were assessed 1 month after dose 1 and 1 and 12 months after dose 2. Reactogenicity and safety were assessed in the total vaccinated cohort through 12 months after dose 2.
Results
There were 232 participants in the total vaccinated cohort, 185 participants in the according‐to‐protocol cohort for humoral immunogenicity, and 58 participants in the according‐to‐protocol cohort for cell‐mediated immunogenicity. Postvaccination anti‐gE antibody concentrations, gE‐specific CD4+ T cell frequencies and VRRs were higher in RZV recipients than in placebo recipients. Solicited adverse events (AEs) were more frequent among RZV recipients than placebo recipients. Incidence of unsolicited AEs, serious AEs, fatalities, and potential immune‐mediated diseases were similar between RZV and placebo recipients.
Conclusion
RZV was immunogenic in patients with STs receiving immunosuppressive chemotherapies. Humoral and cell‐mediated immune responses persisted 1 year after vaccination. No safety concerns were identified.
Keywords: herpes zoster vaccine, immunogenicity, safety, patients with solid tumors, immunosuppressive chemotherapy
Short abstract
In patients with solid tumors treated with immunosuppressive chemotherapies, the adjuvanted recombinant zoster vaccine (RZV) was immunogenic and immunogenicity persisted through 1 year after vaccination. RZV was well tolerated, and no safety concerns were identified.
Introduction
Most adults worldwide show serological evidence of previous varicella zoster virus (VZV) infection.1 Reactivation of latent VZV leads to herpes zoster (HZ), a usually painful, unilateral, vesicular dermatomal rash. The most common complication of HZ is postherpetic neuralgia; a chronic pain that can last for months or even years after the rash has resolved.2, 3
Although age is the risk factor most commonly associated with developing HZ, an increased incidence of HZ has also been associated with immunosuppression induced by certain diseases and/or immunosuppressive treatment. Such conditions include solid tumors (STs), hematologic malignancies (ie, lymphoma), iatrogenic immunosuppression, human immunodeficiency virus (HIV) infection, and certain autoimmune diseases.4, 5, 6, 7, 8, 9, 10 The incidence rate of HZ in individuals with STs receiving immunosuppressive chemotherapy is estimated to be 3‐4 times higher (12/1000 person‐years)11 than in the overall population (3.2/1000 person‐years).12 Additionally, HZ episodes in immunocompromised people can be atypical (disseminated, multidermatomal, involve additional organs), more severe, and/or longer. This leads to increased morbidity in patients already bearing a high disease burden from the underlying malignancy and its treatment. The increased incidence and severity of HZ in these populations is likely due to suppression of their cellular immunity through radiation and/or immunosuppressive chemotherapy.
Due to the increased incidence and severity of common infectious diseases in oncology patients receiving immunosuppressive agents, vaccination against the causal pathogens has been increasingly encouraged. However, several questions remain on the optimal timing and dosage of vaccines to maximize benefit for these patients.13, 14, 15, 16, 17
A live‐attenuated VZV vaccine (ZVL [Zostavax, Merck Sharp & Dohme])18 and an adjuvanted recombinant zoster vaccine (RZV [Shingrix, GSK])19 are licensed for the prevention of HZ in adults ≥50 years of age. In contrast to RZV, ZVL is contraindicated in persons with immunodeficiency or immunosuppression due to disease or immunosuppressive therapy as live‐virus vaccines can cause severe or fatal reactions in immunosuppressed persons due to uncontrolled replication of the vaccine virus.18, 20, 21, 22, 23
A candidate inactivated zoster vaccine (ZVIN) evaluated in immunocompromised adults and adult autologous hematopoietic stem cell transplant (HSCT) recipients has been shown to be generally safe and immunogenic when administered in a 4‐dose schedule over 4 months.24, 25, 26 In the autologous HSCT recipients, the candidate ZVIN vaccine was 64% efficacious in preventing confirmed cases of HZ.27
RZV is a vaccine consisting of the truncated form of VZV glycoprotein E (gE) and the AS01B adjuvant system and is licensed as a 2‐dose schedule in adults ≥50 years of age.19 In phase 3 clinical studies in immunocompromised adults, this 2‐dose schedule was completed in 1‐2 months.28 In adults ≥50 years of age, RZV elicited robust humoral and cell‐mediated immune responses and was >90% efficacious against HZ.29, 30, 31 In addition, RZV was highly immunogenic and well tolerated in autologous HSCT recipients ≥18 years of age and HIV‐infected adults ≥18 years of age.32, 33 In autologous HSCT recipients, RZV was 68% efficacious in preventing HZ.28 In this study, we evaluated the immunogenicity and safety of RZV administered before or at the start of a chemotherapy cycle in adults ≥18 years of age with STs.
Patients and Methods
Study Design
This was a phase 2/3 observer‐blind, randomized, placebo‐controlled, multicenter, multicountry study conducted in Canada, the Czech Republic, France, the Republic of Korea, Spain, and the United Kingdom between March 2013 and May 2016.
Patients with STs were randomized (1:1) using a web‐based central randomization system (SBIR, GSK) to receive 2 doses of RZV or placebo 1‐2 months apart at visits designated M0 and M1. RZV/placebo compositions are described in the Supporting Information. Participants were stratified (4:1) according to the timing of the first RZV or placebo dose with respect to the start of the first (or occasionally second) cycle of a chemotherapy course: first vaccination 8‐30 days before the start of a cycle (RZV‐PreChemo, Placebo‐PreChemo) or first vaccination within 1 day of the start of a cycle (RZV‐OnChemo, Placebo‐OnChemo) (Fig. 1). Participants received their second vaccination with a subsequent chemotherapy cycle. The overall ratio of these 4 study groups—RZV‐PreChemo, Placebo‐PreChemo, RZV‐OnChemo, and Placebo‐OnChemo—was 4:4:1:1. The randomization algorithm used a minimization procedure accounting for age (18‐49 years and ≥50 years), study site, country, and sex. The first vaccination at M0 (visit 1) was preceded by a mandatory prevaccination visit that took place within 30 days before visit 1 or on the same day as visit 1.
Figure 1.
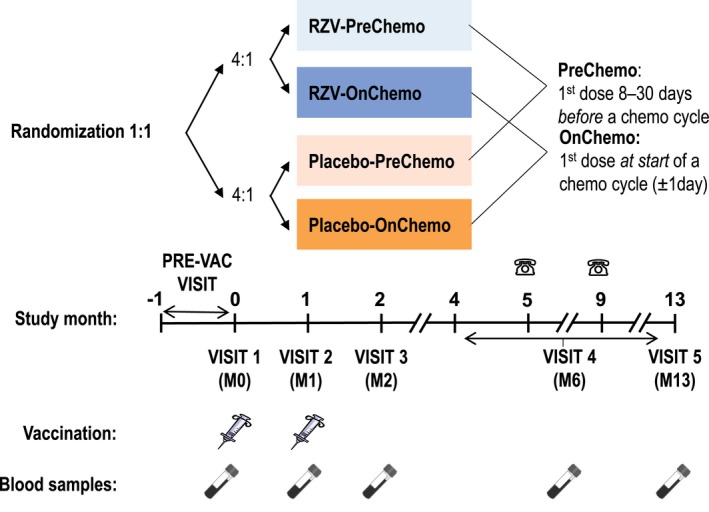
Study design for participants receiving the adjuvanted recombinant zoster vaccine (RZV). RZV or placebo was administered in the deltoid muscle of the nondominant arm unless clinically contraindicated (eg, previous surgical resection of the axillary lymph nodes of the nondominant arm as part of solid tumor management). Syringes represent vaccinations (RZV or placebo doses 1 and 2), test tubes represent blood sampling, and phones represent phone contact. Prevaccination (PRE‐VAC) visit occurred at or within 30 days before the day of visit 1. Visit 1 occurred 8‐30 days before the start of a chemotherapy cycle (RZV‐PreChemo, Placebo‐PreChemo) or at the start of a chemotherapy cycle (RZV‐OnChemo, Placebo‐OnChemo). Visit 2 occurred 1‐2 months after the first vaccination and at the first day (allowing a window of ±1 day) of a subsequent cycle of chemotherapy. Visit 3 occurred approximately 1 month after the second vaccination. Visit 4 occurred within months 4‐13 (at least 2 months after visit 3) at the start of the last cycle of chemotherapy and coincided with the patient’s lowest immune status. Visit 4 could replace month 5 or month 9 phone contacts (in case it coincided with the timing of these) or could coincide with Visit 5 (if the last chemotherapy cycle occurred at month 13). M indicates month.
The study protocol was reviewed and approved by national or regional independent ethics committees or institutional review boards. The study was conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice. The study is registered on ClinicalTrials.gov (NCT01798056). Anonymized individual participant data and study documents can be requested for further research at www.clinicalstudydatarequest.com.
Study Participants
Patients ≥18 years of age who had been diagnosed with 1 or more STs were considered eligible for participation if they were receiving or scheduled to receive cytotoxic or immunosuppressive chemotherapy. Detailed inclusion/exclusion criteria are presented in the Supporting Information.
Written informed consent was obtained from all patients before randomization.
Assessments
Assessment of immunogenicity
Humoral immune responses were evaluated by measuring the anti‐gE antibody concentration31 from blood samples collected at visits 1‐5 from all patients (Fig. 1). Cell‐mediated immunogenicity (CMI) was evaluated from blood samples collected at visits 1‐3, and 5 from a CMI subcohort, by measuring the frequencies of gE‐specific CD4[2+] T cells31 (CD4+ T cells expressing at least 2 of the following 4 activation markers: interferon‐γ, interleukin‐2, tumor necrosis factor α, and CD40 ligand).
Assessment of safety
Diary cards were provided to participants to record solicited adverse events (AEs) for 7 days (D0‐D6) and unsolicited AEs for 30 days (D0‐D29) after each vaccination. Solicited AEs were recorded as local (injection site pain, redness, and swelling) or systemic (fever [oral/axillary/tympanic temperature ≥37.5°C], headache, fatigue, gastrointestinal symptoms [nausea, vomiting, diarrhea and/or abdominal pain], myalgia and shivering). All AEs (except fever) were graded on a scale from 1 (mild) to 3 (severe as defined in the Supporting Information).
All solicited local AEs were considered causally related to vaccination. The causality of all other AEs was assessed by the investigator.
Serious AEs (SAEs), potential immune‐mediated diseases, and intercurrent medical conditions (clinical events during the course of the study that confounded the interpretation of the immunologic assessments, such as HZ, protein losing enteropathy, proteinuria, or cachexia) were reported during the entire study. Suspected HZ cases, as based on investigator clinical judgment, and HZ complications were recorded during the entire study as AEs or SAEs, as appropriate.
Outcomes
Study objectives and their success criteria are summarized in Figure 2. Using a hierarchical procedure, the confirmatory objectives were assessed sequentially in ascending order until an objective was not met, and the remaining objectives were analyzed descriptively.
Figure 2.
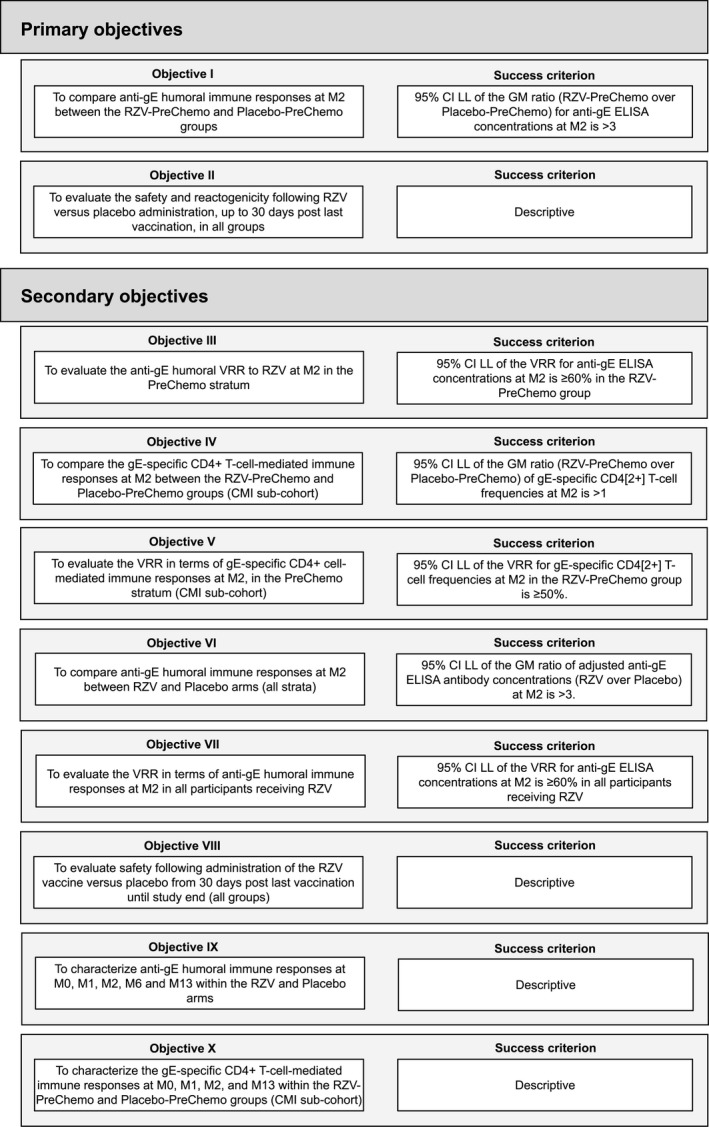
Objectives of the study. In both the PreChemo (first vaccination 8‐30 days before the start of a chemotherapy cycle) and OnChemo (first vaccination at the start of a chemotherapy cycle) strata, the second vaccination took place 1‐2 months after the first vaccination and at the first day (allowing a window of ±1 day) of a subsequent cycle of chemotherapy. CI, confidence interval; CMI, cell‐mediated immunogenicity; ELISA, enzyme‐linked immunosorbent assay; gE, glycoprotein E; GM, geometric mean; LL, lower limit; M, study month; RZV, recombinant zoster vaccine; VRR, vaccine response rate.
Statistical Analyses
The analysis of reactogenicity and safety was performed on the total vaccinated cohort, which included participants who received at least 1 vaccine dose. The analysis of humoral immunogenicity during the vaccination (up to M2) and at persistence phases (up to M13) were performed on the applicable according‐to‐protocol cohorts and included all participants from the PreChemo and OnChemo strata who complied with the protocol‐specified procedures and for whom data were available. The analysis of gE‐specific CMI during the vaccination and persistence phases were performed within the applicable according‐to‐protocol cohorts of the CMI subcohort, which includes randomly selected patients from the PreChemo stratum of selected sites with access to a GSK‐validated peripheral blood mononuclear cells processing facility.
Detailed statistical considerations for the assessment of immunogenicity, as well as sample size considerations, are described in the Supporting Information.
Results
Study Participants
A total of 262 (RZV, n = 130; placebo, n = 132) patients with STs were enrolled and randomized to 1 of the study groups. Of these, 232 (RZV, n = 117; placebo, n = 115) received at least 1 dose, 209 (RZV, n = 102; placebo, n = 107) completed the vaccination phase of the study up to M2, and 180 (RZV, n = 90; placebo, n = 90) completed the study up to the end of the persistence phase at M13 (Fig. 3). Demographic characteristics were comparable between study arms. Among RZV and placebo recipients, respectively, 59.8% and 60.0% were women, and the mean ages of participants included in the total vaccinated cohort were 57.1 and 58.5 years at first vaccination. The most common diagnoses were breast cancer (45.3% and 45.2%) and colorectal cancer (21.4% and 19.1%) in the RZV and placebo arms, respectively (Table 1).
Figure 3.
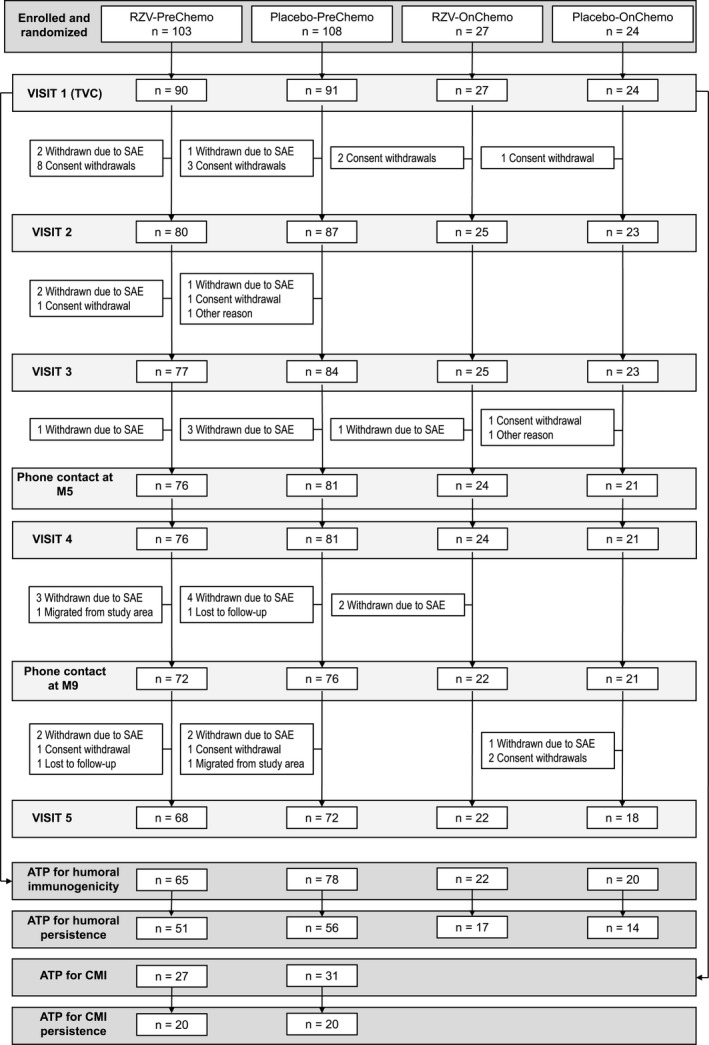
Participant flow. In both the PreChemo (first vaccination 8‐30 days before the start of a chemotherapy cycle) and OnChemo (first vaccination at the start of a chemotherapy cycle) strata, the second vaccination took place 1‐2 months after the first vaccination and at the first day (allowing a window of ±1 day) of a subsequent cycle of chemotherapy. Some participants who were randomized to the PreChemo stratum and were required to wait 8‐30 days after vaccination before they could start chemotherapy chose to withdraw so as to proceed to chemotherapy immediately. ATP, according‐to‐protocol; CMI, cell‐mediated immunogenicity; M, month; n, number of patients attending a visit; pIMD, potential immune‐mediated disease; RZV, recombinant zoster vaccine; SAE, serious adverse event; TVC, total vaccinated cohort.
Table 1.
Summary of Demographic and Disease Characteristics (Total Vaccinated Cohort)
| Characteristics | RZV (N = 117) | Placebo (N = 115) |
|---|---|---|
| Age at visit 1, y, mean ± SD | 57.1 ± 10.8 | 58.5 ± 11.7 |
| Age group, n (%) | ||
| 18‐49 y | 31 (26.5) | 30 (26.1) |
| ≥50 y | 86 (73.5) | 85 (73.9) |
| Sex, n (%) | ||
| Men | 47 (40.2) | 46 (40.0) |
| Women | 70 (59.8) | 69 (60.0) |
| Geographic ancestry, n (%) | ||
| African heritage/African American | 2 (1.9) | 2 (1.9) |
| American Indian/Alaskan native | 2 (1.9) | 0 (0.0) |
| Asian: East Asian heritage | 11 (10.2) | 14 (13.1) |
| Asian: Southeast Asian heritage | 0 (0.0) | 2 (1.9) |
| White: Arabic/North African heritage | 1 (0.9) | 0 (0.0) |
| White: Caucasian/European heritage | 92 (85.2) | 88 (82.2) |
| Other | 0 (0.0) | 1 (0.9) |
| Missinga | 9 (—) | 8 (—) |
| Solid tumor diagnosis, n (%) | ||
| Breast | 53 (45.3) | 52 (45.2) |
| Colorectal | 25 (21.4) | 22 (19.1) |
| Lung | 8 (6.8) | 13 (11.3) |
| Prostate | 5 (4.3) | 4 (3.5) |
| Bladder | 1 (0.9) | 4 (3.5) |
| Pancreas | 1 (0.9) | 1 (0.9) |
| Melanoma | 1 (0.9) | 0 (0) |
| Otherb | 23 (19.7) | 19 (16.5) |
| ECOG performance status, n (%) | ||
| 0: Fully active | 95 (83.3) | 86 (74.8) |
| 1: Restricted in physically strenuous activity | 18 (15.8) | 28 (24.3) |
| 2: Ambulatory and capable of all self‐care | 1 (0.9) | 1 (0.9) |
| Missing | 3 (—) | 0 (—) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; N, total number of participants; RZV, adjuvanted recombinant zoster vaccine; SD, standard deviation.
Missing geographic ancestry information in compliance with local laws preventing the collection of these data.
Includes gastric, endometrial, ovarian, head and neck, larynx, mouth, sinus, tonsil, liposarcoma mixoid, liver, oesophageal, renal, sarcoma, stomach, testicular embryonic carcinoma, thyroid, tongue, cervix, urothelial, and uterine leiomyosarcoma.
Immunogenicity Results
Before vaccination, >99% of participants were seropositive for anti‐gE antibody (data not shown). The anti‐gE antibody geometric mean concentrations (GMCs) ranged between 868.2 and 1185.4 mIU/mL across the 4 study groups (Fig. 4A). In placebo recipients, there was no significant change in GMC after vaccination. At M6, a single placebo recipient (2.4% [95% confidence interval [CI] 0.1%‐12.6%]) who did not report clinical symptoms of HZ met the definition of vaccine response. No other placebo recipients met the criterion for humoral response at any time point (Fig. 4A,B).
Figure 4.
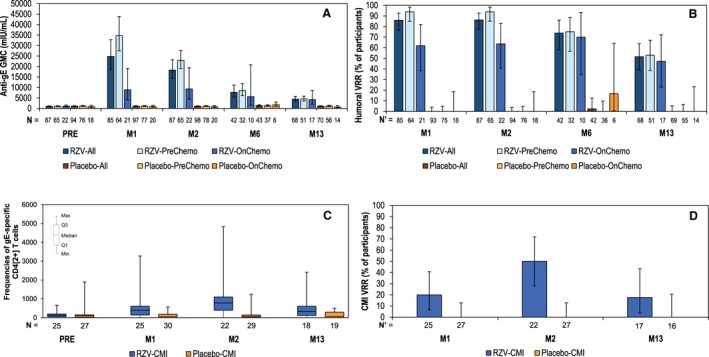
Humoral and cell‐mediated immunogenicity (CMI) of recombinant zoster vaccine (RZV). (A) Anti‐gE antibody GMCs (according‐to‐protocol [ATP] cohort for humoral immunogenicity/persistence). (B) Vaccine response rate (VRR) in terms of anti–glycoprotein E (gE) antibody concentrations (ATP cohort for humoral immunogenicity/persistence). (C) Frequencies of gE‐specific CD4[2+] T cells per 106 total CD4+ T cells (ATP subcohort for CMI, PreChemo groups only). (D) VRR in terms of CMI responses (ATP subcohort for CMI, PreChemo groups only). In both the PreChemo (first vaccination 8‐30 days before the start of a chemotherapy cycle) and OnChemo (first vaccination at the start of a chemotherapy cycle) strata, the second vaccination took place 1‐2 months after the first vaccination and at the first day (allowing a window of ±1 day) of a subsequent cycle of chemotherapy. gE, glycoprotein E; GMC, geometric mean concentration; M, study month; M1, 1 month post‐dose 1; M2, 1 month after dose 2; M6, 5 months after dose 2; M13, 12 months after dose 2; N, number of participants with available results; N’, number of participants with pre‐ and post‐vaccination results available; PRE, prevaccination (M0). With the exception of panel C, error bars indicate 95% confidence intervals.
At M1, in pooled RZV recipients, the GMC was 24793.1 mIU/mL; both GMCs and humoral vaccine response rates (VRRs) were higher in RZV‐PreChemo than in the RZV‐OnChemo group (Fig. 4A,B).
At M2, the adjusted GMC ratio (RZV over placebo) was 23.2 (95% CI, 17.9‐30.0; P < .0001) in the PreChemo stratum; therefore, the first study objective was met. When pooling both the PreChemo and OnChemo strata, the adjusted GMC ratio (RZV over placebo) was 14.4 (95% CI, 10.7‐19.5; P < .0001). In pooled RZV recipients, the M2 GMC was 18291.7 mIU/mL and remained higher in the RZV‐PreChemo group than in the RZV‐OnChemo group (Fig. 4A). The humoral VRR to RZV was 93.8% (95% CI, 85.0%‐98.3%) at M2 in the RZV‐PreChemo group; therefore, the third study objective was met. The humoral VRR was higher in the RZV‐PreChemo group than in the RZV‐OnChemo group (Fig. 4B).
At M13, the GMC was 4477.3 mIU/mL in pooled RZV recipients; both GMCs and humoral VRRs were similar in the RZV‐PreChemo group and RZV‐OnChemo group (Fig. 4A,B).
In RZV recipients ≥50 years of age, GMCs tended to be higher at M2, M6, and M13 than in those 18‐49 years of age, while humoral VRR tended to be higher only at M6 and M13 (Supporting Fig. S1).
Before vaccination, median CD4[2+] T cell frequencies were 127.3 and 104.8 in RZV and placebo recipients, respectively; no increases were observed in placebo recipients at any time point (Fig. 4C).
At M2, the GM ratio of adjusted gE‐specific CD4[2+] T cell frequencies (RZV over placebo) was 9.94 (95% CI, 3.63‐27.19; P < .0001) in the CMI subcohort; therefore, the fourth study objective regarding gE‐specific CMI responses was met. Also at M2, the CMI VRR was 50.0% (95% CI, 28.2%‐71.8%) in RZV recipients; therefore, the fifth study objective, which required a CMI VRR lower limit (LL) 95% CI threshold of 50%, was not met. Hence, this and all subsequent confirmatory objectives of the study were considered descriptive. Among placebo recipients, there were no responders at any time point (Fig. 4D).
In RZV recipients, the highest median CD4[2+] T cell frequency was observed at M2 (778.8), which persisted above baseline up to M13 (332.9), a level similar to that observed at M1 (391.9). CMI VRRs followed a similar pattern, with the highest VRR at M2 (Fig. 4C,D).
CD4[2+] T cell frequencies were in similar ranges in RZV recipients 18‐49 and ≥50 years of age, whereas CMI VRRs tended to be higher in those 18‐49 years of age at all postvaccination time points (Supporting Fig. S2).
Safety Results
At least 1 solicited local symptom (any grade) was reported by 94 (83.9%) RZV and 7 (6.4%) placebo recipients. Grade 3 solicited local symptoms were reported by 13 (11.6%) RZV recipients and none by placebo recipients. Injection site pain was the most frequently occurring solicited local symptom, reported by 90 (80.4%) RZV and 7 (6.4%) placebo recipients (Fig. 5, Supporting Table S1).
Figure 5.
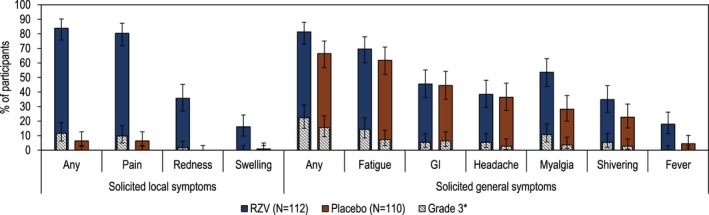
Solicited adverse events (overall/participant, total vaccinated cohort). Error bars indicate 95% confidence intervals. Numerical values for the data depicted here are provided in Supporting Table S1. GI, gastrointestinal symptoms (nausea, vomiting, diarrhea and/or abdominal pain); N, number of participants with ≥1 documented dose; RZV, recombinant zoster vaccine. *Fever was not graded; percentage of participants with oral temperature >39°C are provided in the Grade 3 category.
At least 1 solicited general symptom (any grade) was reported by 91 (81.3%) RZV and 73 (66.4%) placebo recipients. Grade 3 solicited general symptoms were reported by 25 (22.3%) RZV and 17 (15.5%) placebo recipients. The most frequently occurring solicited general symptom after RZV was fatigue, followed by myalgia (Fig. 5, Supporting Table S1).
Solicited local and general symptoms were mild to moderate in intensity and transient, with a median duration up to 4 days.
Unsolicited AEs were reported by 100 (85.5%) RZV and 103 (89.6%) placebo recipients. In each study group, the most frequently occurring unsolicited AEs were nausea and asthenia (weakness), reported by 26.5% and 25.6% of RZV recipients, respectively, and by 24.3% and 24.3% of placebo recipients, respectively. Grade 3 unsolicited AEs were reported by 18 (15.4% [1 case was considered causally vaccine‐related by the investigator]) RZV and 15 (13.0%) placebo recipients (Table 2).
Table 2.
Percentages of Participants Reporting Adverse Events (Total Vaccinated Cohort)
| Adverse Events | RZV (N = 117) | Placebo (N = 115) | ||
|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | |
| Within 30 days after vaccination | ||||
| Unsolicited adverse events | ||||
| Any grade | 100 | 85.5 (77.8‐91.3) | 103 | 89.6 (82.5‐94.5) |
| Grade 3 | 18 | 15.4 (9.4‐23.2) | 15 | 13.0 (7.5‐20.6) |
| Any grade: related | 10 | 8.5 (4.2‐15.2) | 9 | 7.8 (3.6‐14.3) |
| Grade 3: related | 1 | 0.9 (0.0‐4.7) | 0 | 0.0 (0.0‐3.2) |
| MAEs | ||||
| All | 31 | 26.5 (18.8‐35.5) | 33 | 28.7 (20.6‐37.9) |
| First vaccination through 30 days after last vaccination | ||||
| pIMDs | ||||
| All | 0 | — | 0 | — |
| SAEs | ||||
| All | 16 | 13.7 (8.0‐21.3) | 14 | 12.2 (6.8‐19.6) |
| Related | 0 | — | 0 | — |
| 30 days after last vaccination through study end | ||||
| pIMDs | ||||
| All | 0 | 0.0 (0.0‐3.1) | 1 | 0.9 (0.0‐4.7) |
| SAEs | ||||
| All | 30 | 25.6 (18.0‐34.5) | 31 | 27.0 (19.1‐36.0) |
| Related | 0 | — | 0 | — |
| Entire study | ||||
| Fatal SAEs | 12 | 10.3 (5.4‐17.2) | 11 | 9.6 (4.9‐16.5) |
Abbreviations: CI, confidence interval; MAEs, adverse events with medically attended visits; N, total number of participants; n, number of participants with ≥1 documented (solicited adverse events) or administered (other adverse events) dose; pIMDs, potential immune‐mediated diseases; RZV, adjuvanted recombinant zoster vaccine; SAEs, serious adverse events.
Causal relationship to vaccination was assessed by the investigator.
The frequency of SAEs was balanced between study arms across all the periods that were evaluated (Table 2). Overall, the most frequent SAEs reported during the entire study period were infections and infestations, malignant neoplasms, and neutropenia.
Fatal SAEs occurred in 12 (10.3%) of RZV and 11 (9.6%) of placebo recipients. The most frequent fatalities reported by System Organ Class were “neoplasms benign, malignant and unspecified (including cysts and polyps)” followed by “infections and infestations.” None of the SAEs, including fatalities, were considered by the investigators to be causally vaccine‐related (Table 2). In addition to the fatalities, classified as withdrawal, 2 participants withdrew due to nonfatal SAEs: 1 RZV recipient experienced brain metastases and febrile neutropenia, and 1 placebo recipient had pulmonary metastases of prostatic cancer, anemia, bilateral hydronephrosis, and acute renal failure.
During the study, 1 potential immune‐mediated disease was reported (autoimmune thyroiditis), which occurred in a placebo recipient (Table 2).
Suspected HZ cases were reported by 1 RZV recipient at M1 and 2 placebo recipients at M6 and M13, respectively.
Discussion
This study demonstrated that 2 doses of RZV are immunogenic in adults with STs who are receiving immunosuppressive chemotherapies and that immune responses persisted through 12M after dose 2. RZV was well tolerated whether given before or at the start of a chemotherapy cycle (RZV‐PreChemo and RZV‐OnChemo groups, respectively). No RZV‐related safety concerns arose during the study. A results summary with potential clinical relevance is provided in Figure 6 to assist communications to the patient.
Figure 6.
Focus on the patient.
Humoral responses at M2 were higher in the RZV‐PreChemo group than in the RZV‐OnChemo group, likely because RZV‐PreChemo participants had at least 8 days after their first vaccination to develop an RZV immune response before initiation of immunosuppressive chemotherapy, whereas RZV‐OnChemo participants had no immune response window before chemotherapy initiation. Nevertheless, these participants also developed robust RZV humoral immune responses.
In healthy adults receiving 2 doses of RZV, humoral immune responses increase from M1 to M2.31 However, in our ST population, these responses decreased from M1 to M2 in the RZV‐PreChemo group and remained unchanged in the RZV‐OnChemo group. Presumably, this observation is secondary to immunosuppression from chemotherapy administration and the short courses of dexamethasone, which is frequently used to prevent nausea and vomiting. We hypothesized that these immunosuppressive agents impair B cell function and antibody production. Indeed, preclinical studies in dogs support the hypothesis that humoral immune response is lowered by the presence of cancer, compared with healthy matched animals, and that B cell response is further impaired by chemotherapy.34 Immune responses to the recombinant Wilms’ tumor 1 (WT1) protein combined with the immunostimulant AS15 (GSK) were also found to be blunted by concomittant administration of chemotherapy in WT1‐positive breast cancer patients.35
Although the presence of cancer and its immunosuppressive treatment may normally reduce vaccine humoral responses, RZV induces such responses by either treatment schedule of this study. Still, administration of the first RZV dose 8‐30 days before the initiation of a chemotherapy course elicits a stronger humoral response than administration at start of chemotherapy.
Although the anti‐gE antibody GMCs were slightly lower in younger (18‐49 years of age) participants, this finding should be interpreted with caution secondary to the smaller number of younger participants in each RZV group. Indeed, the range of anti‐gE concentrations for the younger and older participants is largely overlapping.
Despite the different immune responses between the RZV‐PreChemo and RZV‐OnChemo groups observed up to M2, vaccine‐induced humoral immunity persisted at similar levels above baseline through M13 for both treatment schedules. Even though administration of the first dose before chemotherapy is desirable, given the higher humoral immunogenicity with this schedule, administration of both RZV doses during chemotherapy still provides persistence of gE‐specific humoral immunity.
VZV‐specific CMI is believed to be the main mechanistic determinant of protection against HZ in older adults35 and may also be the determinant for patients with STs. In contrast to humoral immune responses, CMI responses (PreChemo stratum) increased by administration of the second RZV dose. However, the extent of the protection that may be offered by RZV to ST patients is unclear in the absence of ST efficacy data or an immunologic correlate of protection. In addition, the impact of low cellular response observed in some patients (as evidenced by relatively low CMI VRR at M2 and M13) on protection against HZ is not known. Efficacy trials would be critical to assess the impact of RZV in patients with STs during chemotherapy.
Although of different magnitude than in immunocompetent older adults, the persistence of humoral and CMI responses in patients with STs on chemotherapy are in line with previous findings on the long‐term immunogenicity of RZV.31, 36
The frequency of local injection site reactions was consistent with the reactogenicity profile observed in the previous phase 3 efficacy trials in older adults.29, 30 In contrast, in ST patients, the frequency of solicited general symptoms in both the RZV arm and especially the placebo arm were higher than in immunocompetent older adults, likely reflecting a higher background rate of general symptoms associated with underlying malignancy and treatment.29, 30
The frequencies of unsolicited AEs, medically attended AEs, SAEs, and fatalities were balanced between the RZV and placebo groups, with the type of AEs being primarily consistent with complications of the underlying malignancy and/or its treatment. No suspected HZ cases occurred among RZV recipients who received both vaccine doses.
Our study has several limitations. The sample size was small, and the study was not powered to compare immune responses between the RZV‐PreChemo and RZV‐OnChemo groups, nor was it powered to compare immune responses by age (19‐49 vs ≥50 years of age), cancer type, or chemotherapeutic regimen. Also, the variable intervals between the first dose and the first (occasionally second) chemotherapy cycle in the PreChemo stratum, types of cancer, different stages of disease, different premedication and anti‐emetic treatments, and different type and strength of chemotherapy regimens are all likely to have impacted the immunogenicity results. Larger or more targeted studies would be needed to evaluate this impact in depth. Because patients ≥50 years of age within the PreChemo stratum represent the largest subgroup of the study, this cohort drove the humoral immunogenicity and safety findings. Results from the OnChemo stratum (20% of all participants), the CMI subcohort, the M6 time point, and the 18‐49 years of age group should be interpreted with caution, because the number of evaluable participants in each of these subgroups was small.
In conclusion, this study demonstrates that 2 doses of RZV are immunogenic in patients with STs receiving immunosuppressant chemotherapy regimens. Immunogenicity persisted 12 months after vaccination regardless of the timing of the first vaccination in relation to the start of chemotherapy. Because concomitant chemotherapy may interfere with vaccine immunogenicity, administration of the first dose at least 1 week before initiating chemotherapy is desirable. No vaccine‐specific safety concerns arose during the study.
Funding Support
This work was sponsored by GlaxoSmithKline Biologicals SA, which was involved in all stages of conduct and analysis of the study and covered the costs of development and publishing of this manuscript.
Conflict of Interest Disclosures
Peter Vink, Laura Campora, Emmanuel Di Paolo, Mohamed El Idrissi, Olivier Godeaux, Marta López‐Fauqued, Bruno Salaun, Thomas C. Heineman, and Lidia Oostvogels were employees of the GSK group of companies (GSK) at the time this study was designed, initiated, and/or conducted. Lidia Oostvogels was an employee of CureVacAG as of March 1, 2018, and is an inventor on a patent application related to the vaccine used in this study. Thomas C. Heineman was a paid GSK consultant during manuscript development and is an inventor on a patent application related to the study vaccine. Peter Vink, Laura Campora, Emmanuel Di Paolo, Mohamed El Idrissi, Olivier Godeaux, Bruno Salaun, Thomas C. Heineman, and Lidia Oostvogels hold shares/ stock options in GSK. Hartmut Kristeleit reports fees for consultancy and/or speaker bureau activities from Amgen, Roche, and Eisai. Shelly A. McNeil’s institution has a clinical trial contract with Novartis and has received research grants for conduct of clinical trials by GSK, Merck, Pfizer, and Sanofi Pasteur. Shelly A. McNeil has received honoraria for participation in scientific advisory boards from GSK, Pfizer, Sanofi Pasteur, and Merck and for provision of accredited CME to HCPs on adult immunization and zoster vaccines. Belen Rubio‐Viqueira has received honoraria for scientific advisory board participation from MSD and Lilly and reports personal fees and support from Roche for provision of training and conference attendance. Constanza Maximiano Alonso has received nonfinancial support from Mundipharma, BMS, Pharmamar, Novartis, Janssen‐cilag, MSD, and TEVA and reports personal fees from Sanofi, Pharmamar, Roche, Novartis, Janssen‐cilag, and Bayer. Enrique Grande has received honoraria for ad boards, meetings, and/or lectures from Pfizer, BMS, IPSEN, Roche, Eisai, Eusa Pharma, MSD, Sanofi‐Genzyme, Adacap, Novartis, Pierre Fabre, Lexicon, and Celgene and has received unrestricted research grants from Pfizer, Astra Zeneca, MTEM/Threshold, Roche, IPSEN and Lexicon.
Zostavax is a trademark of Merck Sharpe & Dohme corp. Shingrix is a trademark of the GSK group of companies.
Author Contributions
Peter Vink: Conceptualization, methodology, supervision, visualization, validation, writing (original draft), writing (review and editing). Ignacio Delgado Mingorance, Constanza Maximiano Alonso, Belen Rubio‐Viqueira, Kyung Hae Jung, Juan Francisco Rodriguez Moreno, Enrique Grande, David Marrupe Gonzalez, Sarah Lowndes, Javier Puente, Hartmut Kristeleit, David Farrugia, Shelly A. McNeil: Investigation, project administration, resources, writing (review and editing). Laura Campora, Emmanuel Di Paolo, Marta López‐Fauqued, Bruno Salaun: Formal analysis, validation, writing (review and editing). Mohamed El Idrissi: Methodology, formal analysis, validation, writing (review and editing). Olivier Godeaux: Conceptualization, methodology, supervision, writing (review and editing). Thomas C. Heineman, Lidia Oostvogels: Funding acquisition, conceptualization, methodology, supervision, writing (review and editing).
Supporting information
The Zoster‐028 Study Group collaborators and acknowledgements are listed in the Supporting Information.
The copyright line for this article was changed on 5 July 2019 after original online publication.
References
- 1. Seward J, Jumaan A, et al. VSV: persistence in the population In: Arvin A, Campadelli‐Fiume G, Mocarski,E, et al, eds. Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, UK: Cambridge University Press; 2007. Chapter 40. [Google Scholar]
- 2. Harpaz R, Ortega‐Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57:1‐30. [PubMed] [Google Scholar]
- 3. Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rusthoven JJ, Ahlgren P, Elhakim T, et al. Varicella‐zoster infection in adult cancer patients. A population study. Arch Intern Med. 1988;148:1561‐1566. [PubMed] [Google Scholar]
- 5. Feller L, Wood NH, Lemmer J. Herpes zoster infection as an immune reconstitution inflammatory syndrome in HIV‐seropositive subjects: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:455‐460. [DOI] [PubMed] [Google Scholar]
- 6. Kim SJ, Kim K, Kim BS, et al. Bortezomib and the increased incidence of herpes zoster in patients with multiple myeloma. Clin Lymphoma Myeloma. 2008;8:237‐240. [DOI] [PubMed] [Google Scholar]
- 7. Rodgers S, Leslie KS. Skin infections in HIV‐infected individuals in the era of HAART. Curr Opin Infect Dis. 2011;24:124‐129. [DOI] [PubMed] [Google Scholar]
- 8. Hata A, Kuniyoshi M, Ohkusa Y. Risk of herpes zoster in patients with underlying diseases: a retrospective hospital‐based cohort study. Infection. 2011;39:537‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tong Y, Qian J, Li Y, Meng H, Jin J. The high incidence of varicella herpes zoster with the use of bortezomib in 10 patients. Am J Hematol. 2007;82:403‐404. [DOI] [PubMed] [Google Scholar]
- 10. Gopalan V, Nair RG, Pillai S, Oberholzer T. Genital herpes zoster as a consequence of cancer chemotherapy‐induced immunosuppression: report of a case. J Infect Chemother. 2012;18:955‐957. [DOI] [PubMed] [Google Scholar]
- 11. Habel LA, Ray GT, Silverberg MJ, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:82‐90. [DOI] [PubMed] [Google Scholar]
- 12. Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotton CN, Poznansky MC. Vaccination of oncology patients: an effective tool and an opportunity not to be missed. Oncologist. 2012;17:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackay HJ, McGee J, Villa D, et al. Evaluation of pandemic H1N1 (2009) influenza vaccine in adults with solid tumor and hematological malignancies on active systemic treatment. J Clin Virol. 2011;50:212‐216. [DOI] [PubMed] [Google Scholar]
- 15. Hottinger AF, George AC, Bel M, et al. A prospective study of the factors shaping antibody responses to the AS03‐adjuvanted influenza A/H1N1 vaccine in cancer outpatients. Oncologist. 2012;17:436‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rousseau B, Loulergue P, Mir O, et al. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: the VACANCE study. Ann Oncol. 2012;23:450‐457. [DOI] [PubMed] [Google Scholar]
- 17. Xu Y, Methuku N, Coimbatore P, et al. Immunogenicity of an inactivated monovalent 2009 influenza A (H1N1) vaccine in patients who have cancer. Oncologist. 2012;17:125‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration . Zostavax: highlights of precribing information. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm132831.pdf. Accessed July 1, 2018.
- 19. US Food and Drug Administration . Shingrix: prescribing information. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581605.pdf. Accessed November 9, 2017.
- 20. Arvas A. Vaccination in patients with immunosuppression. Turk Pediatri Ars. 2014;49:181‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ljungman P. Vaccination of immunocompromised patients. Clin Microbiol Infect. 2012;18:93‐99. [DOI] [PubMed] [Google Scholar]
- 22. Alexander KE, Tong PL, Macartney K, Beresford R, Sheppeard V, Gupta M. Live zoster vaccination in an immunocompromised patient leading to death secondary to disseminated varicella zoster virus infection. Vaccine. 2018;36:3890‐3893. [DOI] [PubMed] [Google Scholar]
- 23. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat‐treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis. 2013;208:1375‐1385. [DOI] [PubMed] [Google Scholar]
- 25. Boeckh M, Arvin A, Mullane K, et al. Immunogenicity of inactivated varicella zoster vaccine (ZV(IN)) in autologous hematopoietic stem cell transplant (auto‐HSCT) recipients. Open Forum Infect Dis. 2017;4:S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eberhardson M, Hall S, Papp KA, et al. Safety and immunogenicity of inactivated varicella‐zoster virus vaccine in adults with autoimmune disease: a phase 2, randomized, double‐blind, placebo‐controlled clinical trial. Clin Infect Dis. 2017;65:1174‐1182. [DOI] [PubMed] [Google Scholar]
- 27. Winston DJ, Mullane KM, Cornely OA, et al. Inactivated varicella zoster vaccine in autologous haemopoietic stem‐cell transplant recipients: an international, multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;391:2116‐2127. [DOI] [PubMed] [Google Scholar]
- 28. de la Serna J, Campora L, Chandrasekar P, et al. Efficacy and safety of an adjuvanted herpes zoster subunit vaccine in autologous hematopoietic stem cell transplant recipients 18 years of age or older: first results of the phase 3 randomized, placebo‐controlled ZOE‐HSCT clinical trial [abstract# 11724]. Abstract presented at: BMT Tandem Meetings; February 25th, 2018; Salt Lake City, UT. [Google Scholar]
- 29. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087‐2096. [DOI] [PubMed] [Google Scholar]
- 30. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019‐1032. [DOI] [PubMed] [Google Scholar]
- 31. Cunningham AL, Heineman TC, Lal H, et al. Immune responses to a recombinant glycoprotein e herpes zoster vaccine in adults aged 50 years or older. J Infect Dis. 2018;217:1750‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella‐zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood. 2014;124:2921‐2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berkowitz EM, Moyle G, Stellbrink H‐J, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV‐infected adults: a phase 1/2a randomized, placebo‐controlled study. J Infect Dis. 2015;211:1279‐1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walter CU, Biller BJ, Lana SE, Bachand AM, Dow SW. Effects of chemotherapy on immune responses in dogs with cancer. J Vet Intern Med. 2006;20:342‐347. [DOI] [PubMed] [Google Scholar]
- 35. Weinberg A, Levin MJ. VZV T cell‐mediated immunity. Curr Top Microbiol Immunol. 2010;342:341‐357. [DOI] [PubMed] [Google Scholar]
- 36. Chlibek R, Pauksens K, Rombo L, et al. Long‐term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine. 2016;34:863‐868. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


