Abstract
Current literature and policy in pediatric liver allocation and organ procurement are reviewed here in narrative fashion, highlighting historical context, ethical framework, technical/procurement considerations, and support for a logical way forward to an equitable pediatric liver allocation system that will improve pediatric wait‐list and posttransplant outcomes without adversely affecting adults. Where available, varying examples of successful international pediatric liver allocation and split‐liver policy will be compared to current US policy to highlight potential strategies that can be considered globally. Liver Transplantation 23:86–95 2017 AASLD.
Abbreviations
- AHN
acute hepatic necrosis
- BMI
body mass index
- CTP
Child‐Turcotte‐Pugh
- DCD
donation after cardiac death
- ET
Eurotransplant
- HRQOL
health‐related quality of life
- ICU
intensive care unit
- IFALD
intestinal failure‐associated liver disease
- INR
international normalized ratio
- MELD
Model for End‐Stage Liver Disease
- NSER
nonstandard exception requests
- OPO
organ procurement organization
- OPTN
Organ Procurement and Transplant Network
- PELD
Pediatric End‐Stage Liver Disease
- PICU
pediatric intensive care unit
- UNOS
United Network for Organ Sharing
Adaptation of longterm immunosuppressive regimens and developments in medical care and surgical techniques have contributed to >90% longterm patient and graft survival for pediatric liver transplant recipients. However, several areas of concern remain including mortality on the waiting list in young candidates and variable wait times. As we strive for better quality of survival, pediatric providers are compelled to recognize that mortality is not the only issue of concern as chronically ill children linger on the waiting list. Longterm morbidity of increased pediatric wait‐list times and decreased rates of transplantation may compromise physical, neurological, and social development. Timely transplantation in children will not only reduce mortality but has the potential to improve quality of life and lower societal burden.
Pediatric Liver Wait‐List Morbidity and Mortality
In the United States, mortality of pediatric patients on the liver transplant waiting list persists despite improved care for patients with end‐stage liver disease and directed efforts to increase pediatric priority in liver allocation. Annual pediatric liver wait‐list mortality rates in the United States have ranged from 7% to 12% over the past 5 years.1 Of 581 children on the waiting list in 2013, 541 underwent transplantation, and 49 were removed because of death or being too ill to transplant.1 Pretransplant mortality has improved gradually over time and is now overall currently 6 deaths per 100 wait‐list years. However, the highest mortality rates persist in children under 1 year of age (12.4 deaths per 100 wait‐list years in 2014, with a peak of 41 deaths per 100 wait‐list years in 2005‐2006; Fig. 1). Wait‐list outcomes in neonates and young infants are even worse.2 In 2014, 63% of candidates had been waiting for less than 1 year, but 12.2% had been waiting for 1‐2 years and 24.8% for more than 2 years.1
Figure 1.
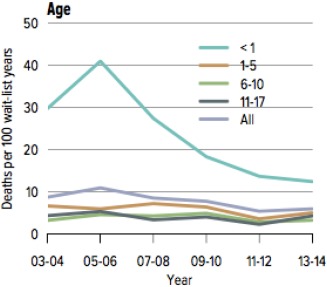
Pretransplant mortality rates among pediatric liver transplant candidates. From Kim et al.1 (2016).
Published information on pediatric transplantation exists for allocation systems in Eurotransplant (ET; which comprises Austria, Belgium, Croatia, Germany, Hungary, the Netherlands, Luxembourg, and Slovenia), Brazil, Australia, and the United Kingdom. However, wait‐list mortality rates have only been described in Brazil and Australia, where rates of pediatric liver wait‐list mortality were 12%‐15% and 6.4%, respectively.3, 4
Morbidity is not robustly described in the pediatric liver wait‐list and posttransplant population. Ng et al.5 demonstrated that only 34% of survivors had an “ideal outcome” (defined by normal clinical and biochemical allograft parameters and absence of immunosuppressive morbidities) at 10 years following transplant. Health‐related quality of life (HRQOL) revealed lower patient self‐reported total scale scores when compared with matched healthy children. Lower HRQOL was associated with prolonged hospitalization at transplant, reoperations, and growth failure at the time of transplant.6 The relationship between these longterm outcomes and increased pediatric wait‐list times has not been specifically explored but are likely to be related because posttransplant impairment is related to the degree of illness at time of transplantation. Wait time and its related comorbidities are not equivalent when comparing a full‐grown adult to a developing child.
Ethical Considerations
Deceased donor livers remain a limited resource, despite the wider utilization of technical variant liver transplants that now make it possible for children to have access to adult deceased donor allografts. This scarcity could be mitigated by increasing rates of living donor liver transplantation or using extended donor criteria to expand the organ pool. Unfortunately, these strategies have had minimal overall impact to date upon wait‐list outcomes in children. Organ allocation systems continue to play a primary role in determining wait time and likelihood of transplantation on the pediatric liver waiting list.
Different pediatric liver allocation systems around the world are subject to a myriad of influences, including but not confined to society, culture, and resources. Caring for children and contributing to their survival is a universal human impulse that was codified by the League of Nations in 1924 in “The Declaration of the Rights of the Child,” which recognized that “mankind owes to the Child the best it has to give,” and accepting it as duty that “beyond and above all considerations of race, nationality or creed…the child must be given the means requisite for its normal development, both materially and spiritually…the child must be the first to receive relief in times of distress.” The Declaration of Geneva, as it came to be known, was adopted unanimously by members of the United Nations General Assembly in 1959.
The United Network for Organ Sharing (UNOS) Pediatric and Ethics Committee created a white paper to support pediatric priority in organ allocation in 2015, entitled “The Ethical Principles of Pediatric Organ Allocation.” This document was broadly written to support pediatric priority across solid organ transplant allocation and highlighted 4 ethical arguments for support: the Prudential Lifespan Account, the Fair Innings Argument, the “Maximin” Principle, and the concept of utility.7 The authors argue for an allocation system for children that not only incorporates wait‐list mortality, but also takes into account the longterm and societal costs incurred from lingering on the waiting list. The Prudential Lifespan Account principle supports a consensus to invest resources across life with the goal of maximizing potential to thrive in the foundational early stages of life. “Fair Innings” reasons that every individual deserves to experience a full life and resources are best used to utilize every opportunity to reach a full life. The “Maximin” principle states that inevitable inequality in allocation should be tempered by a tolerance only when the greatest benefit is given to the least advantaged members of society. Pediatric candidates are vulnerable and disadvantaged and should be conferred priority. The final principle, the concept of utility, is highlighted by the markedly superior 10‐year patient survival of pediatric patients when compared with adults (in liver, 74% versus 56%).7
Procuring the Optimal Liver Graft in Pediatric Transplantation
Outcomes by Graft Type
To a certain point, the availability of multiple graft options for children has resulted in improved wait‐list mortality; several documented series demonstrate excellent outcomes and highlight areas of opportunity that are summarized briefly here.
In a multicenter Organ Procurement and Transplant Network (OPTN) analysis, living donor transplantation has been associated with improved outcomes particularly in the youngest recipients under an age of 2 years.8, 9 Unmatched overall living donor outcomes at 5 and 10 years are incrementally better as compared with deceased donor outcomes in the Scientific Registry of Transplant Recipients database between 1991 and 2013.1, 10 Whole organ transplant overall has been demonstrated to have improved outcomes in overall pediatric cohorts11, 12 as compared with technical variant grafts, but availability of these pediatric donor organs varies by regional supply or allocation policy, particularly in Western countries.13 Other data have demonstrated similar 10‐year patient and graft outcomes across all graft types in children, which underscores the importance of center experience and technical expertise.14
Expanding the Pediatric Donor Pool: Technical Options AND Extended Donor Criteria
Practice in selection of organ type in pediatric liver transplantation varies regionally and is dependent on organ availability as well as legal and medical practice and expertise. Extensive experience in living donor transplantation in children was borne out of necessity in East Asian countries (Japan, Korea) and in South America out of poor or irregular access to deceased donation. The European experience has focused primarily on technical variant grafts as a means to improve organ utilization. Recent US data indicate whole livers were used in 64% of pediatric liver transplants between 2011 and 2013 with an additional 26.5% partial or split grafts and 9.5% living donor grafts.10 Living donor grafts comprised between 7.8% and 10% of the pediatric liver transplants performed between 2011 and 2013; this number has decreased somewhat from an average of 14.9% between 2001 and 2003. This decline in living donor liver transplants in the United States has not been fully explained but may be temporally linked to a high‐profile donor death in 2002.15 Highly publicized coverage of rare donor deaths and risk adverse center practice may have tempered the subsequent rise in the rate of US living liver donation.
The variation in use of whole livers, split grafts, and living donor grafts in the United States also is clearly multifactorial and in part can be explained by regional variation in availability of pediatric whole grafts, center experience in split grafts or living donors, and concern over impact on recipient outcomes. As long as wait‐list mortality persists, however, a need exists to use all available methods, especially technical variant grafts to resolve this issue. US and international data now suggest equivalent outcomes for technical variant grafts that should drive increased utilization of split‐liver transplantation. Despite evidence that the learning curve can be overcome, increased cooperation across centers is still needed as well as networks that can support training and share experience. For example, recent US OPTN analyses demonstrate improved outcomes nationally among pediatric recipients of deceased donor split grafts15 as well as equivalent outcomes among adult recipients of split grafts as compared with whole grafts.16 Finally, in the United Kingdom, an intentional center policy focus on splitting livers resulted in 65% of pediatric LTs being done as split deceased donor grafts with excellent outcomes for both the left lateral pediatric recipient as well as the adult right lobe recipient.17 Most significantly, pediatric wait‐list mortality was eliminated during the last 4 years of the study period.
Assessing Pediatric Liver Graft Quality
Assuring that all viable grafts are maximally used is an ongoing priority in the clinical and research community. Overall, in the United States, liver graft utilization demonstrates an opportunity for improving utilization of marginal grafts. In 2014, for example, of 8594 deceased donor liver grafts, 74.3% underwent transplantation, 12.8% were not recovered primarily for reasons of graft quality, and 7.9% were recovered but not used primarily for histologic or anatomic reasons. The remaining 5% encompassed donors in whom the liver was recovered, but not for transplant, or where consent was either not requested or not obtained.18
Criteria for pediatric liver allograft quality have not been robustly evaluated. In the United States, UNOS currently adopted criteria for voluntary consideration of “splitting a liver” in donors under the age of 40 years, on a single vasopressor and with serum transaminases <3 times the upper limit of normal, and with body mass index (BMI) of < 28 kg/m2. United Kingdom criteria for splittable livers are brain dead donors of an age of <40 years, weighing >50 kg, with <5‐day intensive care unit (ICU) history. As noted above, voluntary splitting of livers to 2 recipients is rare in the United States and essentially only occurs when the organ is first allocated to a pediatric recipient.
Limited data exist on the use of extended criteria donors for children receiving liver transplants. Hypernatremia has not been well studied in liver donors for pediatric recipients. In 1 small series, 4 out of 5 grafts with peak Na between 172 and 193 mEq/L functioned well in the immediate postoperative period.19 Although donor macrosteatosis has been associated with poor outcomes in adult transplantation,20, 21 use of adult donors with BMI between 25 and 35 kg/m2 did not affect pediatric outcomes after deceased donor or living related transplant in a recent OPTN analysis.22 Donors at extremes of age have not been extensively studied. In 1 early study, neonatal donors were associated with higher hepatic arterial thrombosis rate but statistically similar survival rates.23 Older donors (age of >48‐50 years) have been successfully used for split grafts for children but have been associated with intrahepatic strictures24 and prolonged cholestasis.25 Darius et al.,26 however, reported no association with donor age and biliary complications in use of split grafts from donors over the age of 50 years.
Donation after cardiac death (DCD) donors may present an untapped opportunity for transplanting children; the emergence of pediatric data differs from adult experience.27 In 1 recent series using 7 DCD donors age <45 years, BMI <30 kg/m2, <5 day hospitalization, serum liver transaminase levels less than twice the upper limit of normal at time of organ acceptance, and donor warm ischemia under 30 minutes were compared to case‐controlled non‐DCD. At a median follow‐up of 4.5 years, pediatric patient and graft survival as well as incidence of ischemic cholangiopathy did not differ among groups.28
Pediatric Liver Allocation Models
Allocation in the United States
Liver allocation in the United States has evolved significantly since Starzl performed the first successful liver transplant in 1967. Until 1984, allocation in the United States existed without government oversight and involved the local surgeon and care team alongside an organ procurement organization (OPO). Hospitals shared organs on a voluntary basis within a nonformal structure.29 Between 1984 and 2002, which is referred to as the pre–Model for End‐Stage Liver Disease (MELD) era, deceased donor livers were allocated based on hospitalized status. Prior to 1997, those on the waiting list were prioritized within their local OPO based on 2 criteria:
1. Their location (different permutations of hospitalized, in ICU, or home).
2. Accrued waiting time.
This prompted physicians to list patients with liver disease as early as possible to gain advantage in allocation. In 1998, this listing stratification was altered to incorporate the Child‐Turcotte‐Pugh (CTP) score, an indexed score based on 3 objective criteria (total bilirubin, albumin, and international normalized ratio [INR]) and 2 subjective criteria (ascites, encephalopathy) that predicted progression of disease severity. Those in the ICU with acute liver failure retained the highest priority for transplantation, in accordance with their risk of mortality. Pediatric patients with chronic liver disease hospitalized in the ICU were given the same high‐priority status as fulminant hepatic failure patients. Pediatric patients hospitalized (not in the ICU) with chronic liver disease were designated status 2B, and those pediatric patients not hospitalized with chronic liver disease were designated status 3.30 Within these broad 3 strata, significant geographic disparity of outcomes and transplantation rates remained. In response to this crisis, the US Congress issued the “Final Rule” in 1999, mandating that an objective ranking of wait‐list patients be established.31
The Institute of Medicine provided recommendations on how best to implement the Final Rule within the liver allocation system. On February 27, 2002, the CTP stratification system was replaced with the MELD and Pediatric End‐Stage Liver Disease (PELD) scores (Table 1). The MELD scoring system was based on an objective formula that accurately predicted mortality in adults waiting for liver transplantation,33 and it is calculated using the parameters of total bilirubin, INR, and creatinine, with a maximum allowable score of 40. The PELD score was developed separately using a cohort of 884 patients of 0‐17 years of age with chronic liver disease in the Studies for Pediatric Liver Transplantation database, predicting with reasonable precision a composite outcome of “death, transplantation, or admission to the intensive care unit”34 and is based on total bilirubin, INR, albumin, with presence/absence of growth failure and age <1 year, and has no upper maximum.
Table 1.
MELD/PELD Calculator Documentation
| Formulaa | |
|---|---|
| PELD Score |
= 0.480 × loge (bilirubin mg/dL) +1.857 × loge (INR) −0.687 × loge (albumin g/dL) +0.436 if the patient is less than 1 year old +0.667 if the patient has growth failure |
| MELD Score |
= 0.957 × loge (creatinine mg/dL) + 0.378 × loge (bilirubin mg/dL) + 1.120 × loge (INR) + 0.643 |
NOTE: See UNOS/OPTN.32
Multiply the score by 10 and round to the nearest whole number.
In the current US liver allocation system, patients on the pediatric liver transplant waiting list are listed with a designation of status 1A, status 1B, or with a MELD/PELD score. Patients who are at the highest risk of mortality (ie, acute liver failure, hepatic artery thrombosis, or primary nonfunction) maintain highest priority on the waiting list with a status 1A designation. Status 1B includes those patients with standardized exceptions and critically ill patients on ventilator support in the ICU. Patients who do not meet the stringent criteria for status 1A or 1B are listed with a MELD or PELD priority score (MELD score applies to those patients above 12 years of age, PELD to those below 12 years of age).
UNOS recognized at the time of MELD/PELD implementation that the calculated score did not accurately reflect the true mortality risk of every pediatric patient on the waiting list. To address this concern, standardized exceptions were allowed to elevate the priority score in those children who met certain criteria: urea cycle disorders, organic acidemias, hepatoblastoma, cystic fibrosis, and primary hyperoxaluria. Outside of these standardized exceptions, additional exception applications are reviewed on a case‐by‐case basis by UNOS Regional Review Boards.
In 2005, 3 years after implementation of this new allocation system, the pediatric liver transplant community noted an increasing dependence on PELD exception requests for allocation.35 Subsequent examination of nonstandard exception requests (NSER) found a 5‐fold increase since 2002, 93% approval of submitted requests, and variations in approval rates from 65% (region 5) to 100% (region 6).36 NSER approval was significantly associated with decreased risk of pretransplant mortality and increased posttransplant survival; 34% of children listed between 2002 and 2013 for a diagnosis of chronic liver disease had a NSER at the time of transplant. This is worrisome in the face of significant regional and racial variation in rates of NSER—those of white race and private insurance being more likely to benefit; furthermore, having an exception translated into a nearly 3‐fold increased likelihood of transplantation.37
Review of exception narratives and scores requested reveal a wide variation in final scores requested ranging from 15 to 75 (personal communication, UNOS Pediatric Committee Liver Working Group, 2016) and no significant association between clinical factors and the requested score. It is clear that the nonstandard exception process provides de facto pediatric priority and contributes to improved wait‐list and posttransplant survival in certain pediatric patients awaiting liver transplant; it is not clear at what cost this occurs as a result of it not being applied consistently to all children. An ideal solution would allow for pediatric priority to be applied in a uniform fashion rather than a provider‐directed one, reducing both regional and racial disparity.
Finally, significant variation in access to transplantation by US UNOS region has led to efforts to reduce geographical disparity through redistricting. Modeling through liver simulated allocation modeling has shown little significant impact upon pediatric allocation and rates of transplantation.38 Increasing the overall pool and competition for organs will likely lead to more widespread use of NSER and with it, the accompanying disparities in wait‐list and posttransplant outcome.
Global Liver Prioritization and Allocation in Children
In ET, Canada, and Brazil, where use of PELD alone has been deemed inadequate in properly prioritizing children on the pediatric liver waiting list, modifications to MELD/PELD allocation have been introduced in varied permutations in the effort to prioritize children and recognize the necessity for prompt transplantation in children with end‐stage liver disease. A review of these alternative modified uses of PELD/MELD and pediatric priority for children on the liver transplant waiting list can inform the next steps for improving the US pediatric liver allocation system. Pediatric prioritization and split‐liver policy used in ET and selected European countries is shown in Table 2.
Table 2.
Pediatric Prioritization and Split‐Liver Policy in Selected European Countries
| Francea | United Kingdomb | Spainc | Italyd | ETe | Switzerlandf | |
|---|---|---|---|---|---|---|
| Prioritization of pediatric patients | Children have access to emergency status (wait time 0‐6 months) for AHN, emergent retransplantation, hepatoblastoma, acute/chronic decompensation, or metabolic disease after external expert review | Children are prioritized for all donors under age 16 years immediately after super‐urgent local and national patients, hepatoblastoma, and IFALD patients | Children (under age 16 years) listed for emergent retransplantation | Donor livers under age 18 years offered to children first. Single national waiting list with specific allocation rules for pediatric recipients | Children aged 16 years and under receive allocation equivalent to 35% 3‐month mortality with automatic 15% monthly increase | Donor organ < 18 years offered to patients age <18 years by priority score and following sequence: 1. Patient < 12 years 2. Patient 12‐18 years 3. > 18 years of age |
| Split policy | Donors under age 30 years are first proposed to pediatric liver teams | Donor age < 40 years, > 50 kg and < 5 days in PICU | Donors less than age 16 years are offered to children first | Donors age 18‐50 years not allocated to super‐urgent or the MELD > 30 list are offered to pediatric centers to decide split feasibility | Donors under age 50 years and >50 kg are considered splittable livers | Liver can be split if the patient with the highest priority consents to the split |
| Liver disease severity score used | PELD not used | Liver is allocated to center for patient selection | Liver is allocated to center for patient selection | Grafts are allocated among pediatric recipients according to PELD (MELD in adolescents) + exceptions | Liver is allocated to center for patient selection | |
| Additional notes | No prioritization for multivisceral candidates | Approximately 20% donors used are split | <2% livers used are split | Right lobe returned to normal allocation | Right lobe returned to normal allocation | MELD‐based system with exception point accrual for children |
| Additional reference | Agence de la Biomédecine39 | Organ Donation and Transplantation40 | Organización Nacional de Trasplantes41 | Centro Nazionale Trapianti Operativo42 | Eurotransplant43 | Schweizerische Eidgenossenschaft Confédération Suisse44 |
C. Chardot and F. Lacaille, personal communication.
P. McKiernan, personal communication.
J. Bueno, personal communication.
M. Spada and J. de Ville de Goyet, personal communication.
U. Baumann, personal communication.
V. McLin, personal communication.
In the ET countries, instead of using the PELD score for pediatric liver allocation, a “pediatric MELD score” is calculated for each child.45 Priority is maintained for those patients with high‐urgency status, primarily those with acute hepatic failure and acute liver graft failure following liver transplant. In addition, urea cycle defects and nonmetastatic hepatoblastoma patients are assigned high‐urgency status if they have not undergone transplantation within 30 days of listing. The remaining patients are automatically assigned an initial MELD score, a “pediatric MELD score” that is calculated for children under 12 years of age as a point score corresponding to a 35% wait‐list mortality. This score is upgraded every 90 days by an additional 15% increase of 3‐month wait‐list mortality until transplantation. For children 12‐16 years of age, a point score is assigned corresponding to 15% wait‐list mortality and upgraded every 90 days by an additional 10% of 3‐month wait‐list mortality. This pediatric MELD score results solely in an assigned score resulting in identical initial point values that are independent of medical urgency. Introduction of this allocation system in ET countries has led to a clear prioritization of children that has resulted in low wait‐list mortality and good clinical outcomes.46 Likewise in Canada, France, and the United Kingdom, there are systems in place to uniformly prioritize children on the pediatric liver waiting list above adults (F. Lacaille, V. Ng, and P. McKiernan, personal communication). In July 2006, Brazil implemented a system wherein patients of <12 years of age had a final allocation score that was the calculated PELD score multiplied by 3. This led to a 6.1‐fold increase in split‐liver transplantation as well as a statistically significant decreased time on the waiting list.3 Ultimately, an allocation system providing additional pediatric priority is more likely to result in increased liver utilization through the use of technical variant or split grafts. Whole cadaveric livers that are first offered to adults are unlikely to be split. However, livers that are being split for children are more likely to have the remaining segment be allocated to an adult. Modeling to demonstrate additional split volume if children are prioritized will be important. Although it is acknowledged that change in behavior as well as local technical expertise with split transplantation may be difficult to predict, these issues would not be expected to be the limiting factors in improving outcomes on the waiting list.
A Mandate for Improvement
The success of liver transplantation across the globe has made this therapy accessible to all children who await this lifesaving therapy. However, organ scarcity and current allocation systems have introduced new outcome disparities and injustice in access for pediatric patients, which our community must now address. In the United States, pediatric wait‐list volume accounts for approximately 10% of the combined adult and pediatric volume. Eradication of pediatric liver wait‐list mortality is an achievable goal and should be a global mandate. The greater community of those who care for pediatric liver transplant candidates and recipients can focus their advocacy efforts in medical and political arenas to attain this goal. When considering this important goal, however, we must not ignore the ongoing concerns with rising adult wait‐list mortality. It is not likely that offering children timely transplantation through pediatric priority will result in a decreased number of transplants or lost opportunity for adults on the list, because the annual number of pediatric liver transplants has been essentially static over the last 15 years. This review of current pediatric liver wait‐list outcomes and allocation practice, coupled with the ethical principles surrounding the care of children as well as the precedent set by international experience suggests that our efforts should converge around the following focal points:
Prioritization of Access to Liver Transplantation to Children
With the exception of the United States, many other countries (Table 2) have prioritized liver allocation in a definitive manner without detriment to adult liver access. A PELD+ modification such as used in Brazil may incorporate some level of medical urgency into the allocation algorithm. Modeling of solutions can proceed in concert with efforts to eliminate geographic or regional disparity in organ availability and standardize exception scores that take into account the heterogeneity of pediatric liver disease and pediatric risk assessment.
Renewing Emphasis on Splitting Livers for 2 Recipients
Revisiting splitting criteria is critical. Adopting algorithms (Fig. 2) such as those proposed by Hong et al.14 and others and implemented on a limited regional or international basis17 could be expanded and incentivized. The UNOS policy 9.8.A open variance allowing regional splitting when the adult is the index patient has not resulted in a significant increase in split grafts. US pediatric recipients used 15% of deceased donor grafts as split grafts between 2002 and 2004 as compared to 16% between 2012 and 2014.1 This lack of change in split utilization likely suggests pediatric prioritization will be important to drive split application, as has been successfully applied in the United Kingdom.17 Europe has often adopted placement of the right lobe to a secondary recipient according to regular allocation practice suggesting that this model also can be successful.
Figure 2.

Split liver algorithm with pediatric prioritization and incentivized splitting (Hong et al.14 [2009]).
Living Donor Liver Transplantation
Global emphasis should be placed on training and disseminating expertise in living donor liver transplantation to help address mortality from liver disease in areas where deceased donation is not widely available or practical.
Expanding Liver Graft Utilization
Finally, opportunities exist for optimizing utilization of all appropriate liver allografts. Research efforts in organ preservation along with appropriate use of extended criteria donors in pediatric liver transplantation will be important to the overall goal of eliminating pediatric wait‐list mortality and improving outcomes for children after liver transplantation.
Conclusions
Pediatric liver transplantation is a lifesaving therapy with excellent results for children with end‐stage liver disease and other life‐threatening conditions. Access to this therapy worldwide is limited by organ scarcity and quality, which can be overcome with minimal detriment to the adult wait‐list population through increasing pediatric priority and emphasizing the importance of utilization of split grafts. The current national data supporting equivalent outcomes with technical variant grafts can help support center and transplant community action to reduce or eliminate wait‐list deaths, improve time to transplant, and ultimately improve longterm outcomes for children in need of liver transplantation.
Correction statement: The copyright line for this article was changed on July 30, 2019 after original online publication.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. Liver. Am J Transplant 2016;16(suppl 2):69–98. [DOI] [PubMed] [Google Scholar]
- 2. Jimenez‐Rivera C, Nightingale S, Benchimol EI, Mazariegos GV, Ng VL. Outcomes in infants listed for liver transplantation: a retrospective cohort study using the United Network for Organ Sharing database. Pediatr Transplant 2016;20:904–911. [DOI] [PubMed] [Google Scholar]
- 3. Neto JS, Carone E, Pugliese RP, Fonseca EA, Porta G, Miura I, et al. Modified pediatric end‐stage liver disease scoring system and pediatric liver transplantation in Brazil. Liver Transpl 2010;16:426–430. [DOI] [PubMed] [Google Scholar]
- 4. Fink MA, Berry SR, Gow PJ, Angus PW, Wang BZ, Muralidharan V, et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol 2007;22:119–124. [DOI] [PubMed] [Google Scholar]
- 5. Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, et al.; for Studies of Pediatric Liver Transplantation (SPLIT) Research Group . Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr 2012;160:820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alonso EM, Martz K, Wang D, Yi MS, Neighbors K, Varni JW, et al.; for Studies of Pediatric Liver Transplantation (SPLIT) Functional Outcomes Group (FOG) . Factors predicting health‐related quality of life in pediatric liver transplant recipients in the functional outcomes group. Pediatr Transplant 2013;17:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. UNOS/OPTN Pediatric and Ethics Committees . Ethical principles of pediatric organ allocation. https://optn.transplant.hrsa.gov/resources/ethics/ethical-principles-of-pediatric-organ-allocation/. Accessed November 2014.
- 8. Roberts JP, Hulbert‐Shearon TE, Merion RM, Wolfe RA, Port FK. Influence of graft type on outcomes after pediatric liver transplantation. Am J Transplant 2004;4:373–377. [DOI] [PubMed] [Google Scholar]
- 9. Abt PL, Rapaport‐Kelz R, Desai NM, Frank A, Sonnad S, Rand E, et al. Survival among pediatric liver transplant recipients: impact of segmental grafts. Liver Transpl 2004;10:1287–1293. [DOI] [PubMed] [Google Scholar]
- 10. Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 annual data report: liver. Am J Transplant 2015;15(suppl 2):1–28. [DOI] [PubMed] [Google Scholar]
- 11. Martin SR, Atkison P, Anand R, Lindblad AS; for SPLIT Research Group . Studies of Pediatric Liver Transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr Transplant 2004;8:273–283. [DOI] [PubMed] [Google Scholar]
- 12. Jain A, Mazariegos G, Kashyap R, Kosmach‐Park B, Starzl TE, Fung JJ, Reyes J. Pediatric liver transplantation in 808 consecutive children: 20‐years experience from a single center. Transplant Proc 2002;34:1955–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rana A, Kaplan B, Riaz IB, Porubsky M, Habib S, Rilo H, et al. Geographic inequities in liver allograft supply and demand: does it affect patient outcomes? Transplantation 2015;99:515–520. [DOI] [PubMed] [Google Scholar]
- 14. Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, et al. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10‐year comparative analysis of 2,988 cases. J Am Coll Surg 2009;208:682–689. [DOI] [PubMed] [Google Scholar]
- 15. Cauley RP, Vakili K, Potanos K, Fullington N, Graham DA, Finkelstein JA, Kim HB. Deceased donor liver transplantation in infants and small children: are partial grafts riskier than whole organs? Liver Transpl 2013;19:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cauley RP, Vakili K, Fullington N, Potanos K, Graham DA, Finkelstein JA, Kim HB. Deceased‐donor split‐liver transplantation in adult recipients: is the learning curve over? J Am Coll Surg 2013;217:672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Battula NR, Plato M, Anbarasan R, Perera MT, Ong E, Roll GR, et al. Intention to split policy: a successful strategy in a combined pediatric and adult liver transplant center. Ann Surg 2016. [DOI] [PubMed] [Google Scholar]
- 18. Israni AK, Zaun D, Bolch C, Rosendale JD, Snyder JJ, Kasiske BL. Deceased organ donation. Am J Transplant 2016;16(suppl 2):195–215. [DOI] [PubMed] [Google Scholar]
- 19. Uribe M, Alba A, González G, Hunter B, Heine C, Iñiguez R, et al. Pediatric liver transplant outcome using severe hypernatremic donors. Transplant Proc 2013;45:3726–3727. [DOI] [PubMed] [Google Scholar]
- 20. de Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol 2012;27:540–546. [DOI] [PubMed] [Google Scholar]
- 21. Spitzer AL, Lao OB, Dick AA, Bakthavatsalam R, Halldorson JB, Yeh MM, et al. The biopsied donor liver: incorporating macrosteatosis into high‐risk donor assessment. Liver Transpl 2010;16:874–884. [DOI] [PubMed] [Google Scholar]
- 22. Perito ER, Rhee S, Glidden D, Roberts JP, Rosenthal P. Impact of the donor body mass index on the survival of pediatric liver transplant recipients and post‐transplant obesity. Liver Transpl 2012;18:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yokoyama I, Tzakis AG, Imventarza O, Todo S, Casavilla A, Leggio A, Starzl TE. Pediatric liver transplantation from neonatal donors. Transpl Int 1992;5:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lüthold SC, Kaseje N, Jannot AS, Mentha G, Majno P, Toso C, et al. Risk factors for early and late biliary complications in pediatric liver transplantation. Pediatr Transplant 2014;18:822–830. [DOI] [PubMed] [Google Scholar]
- 25. Petz W, Spada M, Sonzogni A, Colledan M, Segalin A, Lucianetti A, et al. Pediatric split liver transplantation using elderly donors. Transplant Proc 2001;33:1361–1363. [DOI] [PubMed] [Google Scholar]
- 26. Darius T, Rivera J, Fusaro F, Lai Q, de Magnée C, Bourdeaux C, et al. Risk factors and surgical management of anastomotic biliary complications after pediatric liver transplantation. Liver Transpl 2014;20:893–903. [DOI] [PubMed] [Google Scholar]
- 27. Yoo PS, Olthoff KM, Abt PL. Donation after cardiac death in pediatric organ transplantation. Curr Opin Organ Transplant 2011;16:483–488. [DOI] [PubMed] [Google Scholar]
- 28. Hong JC, Venick R, Yersiz H, Kositamongkol P, Kaldas FM, Petrowsky H, et al. Liver transplantation in children using organ donation after circulatory death: a case‐control outcomes analysis of a 20‐year experience in a single center. JAMA Surg 2014;149:77–82. [DOI] [PubMed] [Google Scholar]
- 29. Van Meter CH. The organ allocation controversy: how did we arrive here? Ochsner J 1999;1:6–11. [PMC free article] [PubMed] [Google Scholar]
- 30. McDiarmid SV, Davies DB, Edwards EB. Improved graft survival of pediatric liver recipients transplanted with pediatric‐aged liver donors. Transplantation 2000;70:1283–1291. [DOI] [PubMed] [Google Scholar]
- 31. Organ Procurement and Transplantation Network‐‐HRSA . Final rule with comment period. Fed Regist 1998;63:16,296–16,338. [PubMed] [Google Scholar]
- 32. UNOS/OPTN . https://www.unos.org. Accessed August 5, 2016.
- 33. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al.; for United Network for Organ Sharing Liver Disease Severity Score Committee . Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91–96. [DOI] [PubMed] [Google Scholar]
- 34. Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl 2001;7:567–580. [DOI] [PubMed] [Google Scholar]
- 35. Shneider BL, Suchy FJ, Emre S. National and regional analysis of exceptions to the Pediatric End‐Stage Liver Disease scoring system (2003‐2004). Liver Transpl 2006;12:40–45. [DOI] [PubMed] [Google Scholar]
- 36. Braun HJ, Perito ER, Dodge JL, Rhee S, Roberts JP. Nonstandard exception requests impact outcomes for pediatric liver transplant candidates. Am J Transplant 2016;16:3181–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsu EK, Shaffer M, Bradford M, Mayer‐Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transplant 2015;15:436–444. [DOI] [PubMed] [Google Scholar]
- 38. Gentry SE, Massie AB, Cheek SW, Lentine KL, Chow EH, Wickliffe CE, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant 2013;13:2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agence de la Biomédecine. www.agence-biomedecine.fr. Accessed October 2016.
- 40. Organ Donation and Transplantation . Liver Advisory Group Papers. http://www.odt.nhs.uk/transplantation/advisory-groups/liver/papers/. Accessed October 2016.
- 41. Organización Nacional de Trasplantes . www.ont.es. Accessed October 2016.
- 42. Centro Nazionale Trapianti Operativo . Protocollo Sulle Procedure di Split Liver Convenzionale in Ambito Nazionale. http://www.crtsicilia.it/centri_trap/protocollo_split-operativo12032016.pdf. Accessed October 2016.
- 43. Eurotransplant . Eurotransplant Manual. https://www.eurotransplant.org/cms/index.php?page=et_manual. Accessed October 2016.
- 44. Schweizerische Eidgenossenschaft Confédération Suisse . Ordonnance du DFI sur l'attribution d'organes destinés à une transplantation. https://www.admin.ch/opc/fr/classified-compilation/20062074/index.html. Accessed October 2016.
- 45. Herden U, Wischhusen F, Heinemann A, Ganschow R, Grabhorn E, Vettorazzi E, et al. A formula to calculate the standard liver volume in children and its application in pediatric liver transplantation. Transpl Int 2013;26:1217–1224. [DOI] [PubMed] [Google Scholar]
- 46. Herden U, Grabhorn E, Briem‐Richter A, Ganschow R, Nashan B, Fischer L. Developments in pediatric liver transplantation since implementation of the new allocation rules in Eurotransplant. Clin Transplant 2014;28:1061–1068. [DOI] [PubMed] [Google Scholar]


