Abstract
Hydroponic ginseng (HPG) has been known to have various bio-functionalities, including an antioxidant effect. Recently, fermentation by lactic acid bacteria has been studied to enhance bio-functional activities in plants by biologically converting their chemical compounds. HPG roots and shoots were fermented with Leuconostoc mesenteroides KCCM 12010P isolated from kimchi. The total phenolic compounds, antioxidant, anti-inflammatory, and anti-adipogenic effects of these fermented samples were evaluated in comparison with non-fermented samples (control). During 24 h fermentation of HPG roots and shoots, the viable number of cells increased to 7.50 Log colony forming unit (CFU)/mL. Total phenolic and flavonoid contents of the fermented HPG roots increased by 107.19% and 645.59%, respectively, compared to non-fermented HPG roots. The antioxidant activity of fermented HPG, as assessed by 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), β-carotene-linoleic, and ferric reducing antioxidant power (FRAP) assay, was also significantly enhanced. In an anti-inflammatory effect of lipopolysaccharide (LPS)-stimulated RAW 264.7 cells, the nitric oxide content and the expression of inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) decreased when treated with fermented samples. Simultaneously, lipid accumulation in 3T3-L1 adipocyte was reduced when treated with fermented HPG. Fermentation by L. mesenteroides showed improved antioxidant, anti-inflammatory and anti-adipogenic HPG effects. These results show that fermented HPG has potential for applications in the functional food industry.
Keywords: anti-adipogenic effect, anti-inflammatory effect, antioxidative effect, hydroponic ginseng, fermentation, Panax ginseng
1. Introduction
Korean Panax ginseng Meyer has been used as a functional food source. The bio-functionality of ginseng, including antioxidation and immune enforcing properties, is derived from various phytochemicals in ginseng, such as numerous kinds of ginsenosides [1], glycosylic acid, and polyphenolic compounds. It takes several years to cultivate ginseng in soil, but there are several obstacles including diseases as well as the accumulation of residual heavy metals and pesticides during cultivation. Recently, studies on hydroponic ginseng (HPG), which is cultivated in mineral nutrient solutions, has performed increasingly to overcome these obstacles. Additionally, the management cost of a hydroponic cultivation technique is much less time-consuming and more economical than that of the conventional soil-cultivation technique. Very few studies, comparing the functional effects of HPG to those of ginseng cultivated in soil, exist but some researchers reported anti-oxidative and anti-inflammatory properties of hydroponic ginseng and soil-cultured ginseng [2].
Nowadays, bioconversion, also called biotransformation, has been widely applied to plants containing bio-functional chemicals. Bioconversion through fermentation is a well-established traditional technology across history used to enhance nutritional properties. The presence of complex enzymes in lactic acid bacteria (LAB), such as Lactobacillus pp., Pediococcus spp., and Leuconostoc spp., leads to functional benefits, for instance, improving nutritional value, digestibility of foods, and modification of flavor attributes [3,4]
According to a previous study [5], Leuconostoc mesenteroides KCCM 12010P isolated from kimchi has a high level of β-glucosidase activity, which can convert ginsenoside Rb2 to Rd and Rg3. In that study, ginseng extract showed anti-inflammatory and anticancer effects following fermentation by L. mesenteroides. Polyphenol content also can be bioconverted by Lactobacillus plantarum, as in the study of Gan et al. [6], where 9 h of fermentation by L. plantarum WCFS1 increased total polyphenol content and improved the antioxidant capacity in mung bean.
Oxidative stress, caused by free radicals, gives rise to peroxidation of proteins, lipids, and DNA, and ultimately induces inflammatory responses, resulting in chronic diseases [7,8]. Obesity, a major public health problem, is also associated with an inflammatory disorder [9]. Adipocytes do not only store surplus energy, but also play a crucial role in the endocrine and immune systems that regulate human body homeostasis. For example, mature adipocytes can up-regulate the transcriptional regulator, nuclear factor kappa-light-chain-enhancer (NF-κB), leading to the secretion of interleukin-6 (IL-6) and the tumor necrosis factor-α (TNF-α), which moderate immune response [10,11]. Several studies have reported that TNF-α plays a major role in insulin resistance and is associated with obesity, type 2 diabetes and adipocyte differentiation [12]. Previous studies have confirmed the biofunctionality of fermented ginseng, such as its anti-hyperglycemic effect on type 2 diabetes mellitus mice or its inhibitory effect on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells [13,14]. However, we do not know of any studies on hydroponic ginseng, including shoots and roots fermented with lactic acid bacteria.
In this study, each part of hydroponic ginseng was fermented with Leuconostoc mesenteroides KCCM 12010P isolated from kimchi, and the resultant enhanced total polyphenol content and health-improving properties, including antioxidant, anti-inflammatory, and anti-adipogenic effects, were verified.
2. Results and Discussion
2.1. Enzymatic Activity of Leuconostoc Mesenteroides KCCM 12010P
The enzymatic activity of the strain was measured with the API ZYM kit (Biomerieux Inc., Marcy I’Etoile, France), and indicated as 0, 5, 10, 20, or 40 nM as indicated in a manual of the kit. The strain had ˃40 nM β-glucosidase activity, 5 nM esterase, alkaline phosphatase, and acid phosphatase activity, 10 nM leucine arylamidase, Naphthol-ASBI-phosphohydrolase, 20 nM α-galactosidase, and 30 nM α-glucosidase activity in hydrolyzed substrate. However, β-glucuronidase, which is a factor in developing cancer was not detected (Table 1).
Table 1.
Enzymatic activity of Leuconostoc mesenteroides KCCM 12010P using an API kit.
| Enzymes | Activity 1) | Enzymes | Activity |
|---|---|---|---|
| Control | 0 | Acid phosphatase | 5 |
| Alkaline phosphatase | 5 | Naphthol-ASBI-phosphohydrolase | 10 |
| Esterase | 5 | α-Galactosidase | 20 |
| Esterase lipase | 0 | β-Galactosidase | ≥40 |
| Lipase | 0 | β-Glucuronidase | 0 |
| Leucine arylamidase | 10 | α-Glucosidase | 30 |
| Valine arylamidase | 0 | β-Glucosidase | ≥40 |
| Cystine arylamidase | 0 | N-Acetyl-β-glucosaminidase | 0 |
| Trypsin | 0 | α-Mannosidase | 0 |
| α-Chymotrypsin | 0 | α-Fucosidase | 0 |
1) 0, no enzyme activity; 5, 10, 20, 30, and ≥ 40 indicates concentration (nM) of hydrolyzed substrate after 4 h of incubation at 37 °C.
2.2. Changes in the Number of Cells and pH During Fermentation
The viable cell number in HPG roots fermented by L. mesenteroides KCCM 12010P increased from 6.14 to 7.50 Log CFU/ mL during 24 h fermentation, while the microbial growth in fermented HPG shoots increased from 6 to 7.6 Log CFU/ mL (Figure 1). The pH in broth containing 5% HPG root and 5% HPG shoot extracts decreased from 5.95 to 4.73 and 5.82 to 4.39, respectively, which could be attributed to the bioconversion of phytochemicals, such as polyphenols and ginsenosides in the samples [15].
Figure 1.
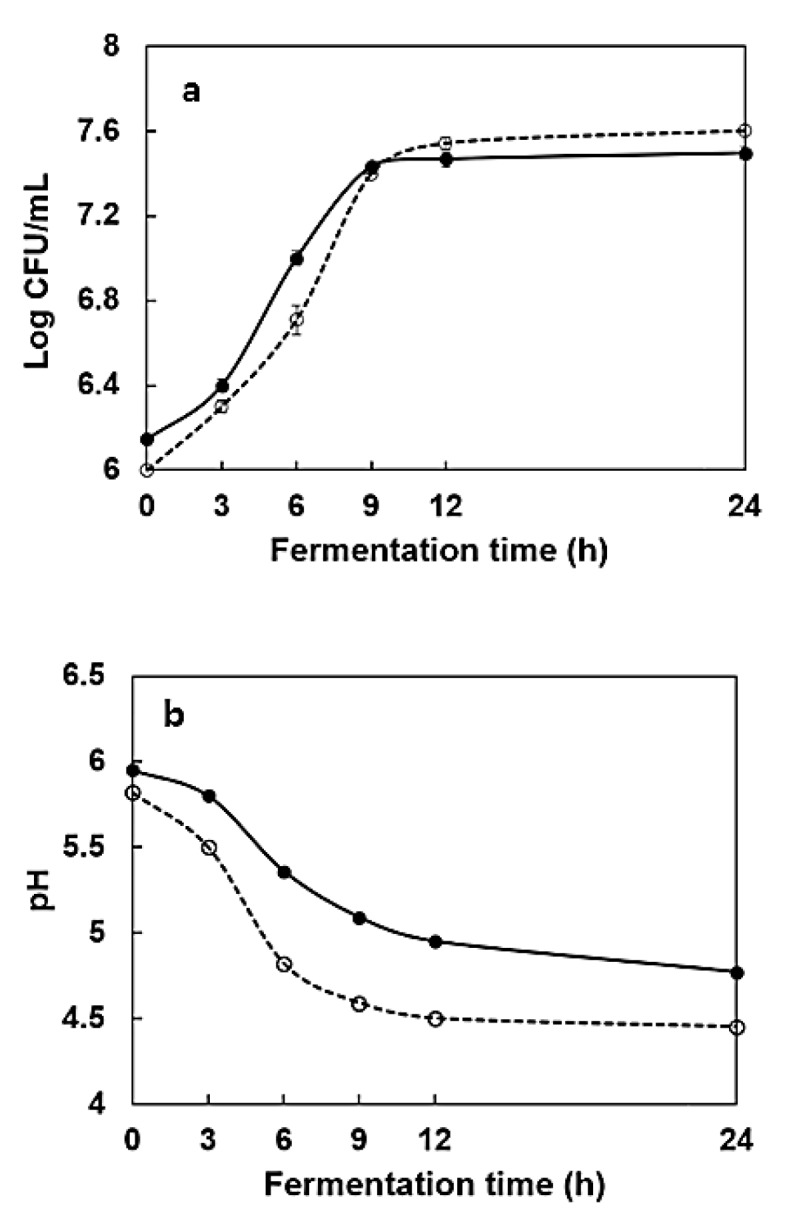
Growth curve (a) and pH (b) changes in hydroponic ginseng root and shoot during 24 h fermentation by Leuconostoc mesenteroides KCCM 12010P. Hydroponic ginseng (HPG) roots (●); HPG shoots (○).
2.3. Total Polyphenol and Flavonoid Content
As shown in Table 2, the total polyphenol content (TPC) and total flavonoid content (TFC) of HPG roots and shoots increased during fermentation. The TPC of fermented HPG roots and shoots increased by 107.19% and 123.19%, respectively, after fermentation. Flavonoid (a kind of polyphenolic compound) contents were also increased by 645.59% and 217.98%, in roots and shoots, respectively. According to a previous study by Rodríguez et al. [16], these results could be due to enzymatic degradation of rutin (quercetin-3-O-rutinoside), which is abundant in ginseng, to a kaempferol-3-rutinoside intermediate during fermentation.
Table 2.
Total polyphenol, flavonoid contents, and antioxidant activities of non-fermented and fermented hydroponic ginseng.
| Sample | Total Polyphenol Content (GAE1)) |
Total Flavonoid Content (KPE2)) |
Antioxidant Activity | |||
|---|---|---|---|---|---|---|
| ABTS Radical Scavenging Activity (%) | FRAP Assay (μM Fe2+/mg Solid Sample) |
Inhibition of β-carotene Oxidation (%) |
||||
| Non- fermented | Roots | 27.96 ± 0.35d | 0.68 ± 0.18d | 34.21 ± 0.36d | 89.47 ± 0.865d | 84.72 ± 0.72b |
| Shoots | 32.56 ± 0.40b | 13.74 ± 0.24b | 54.69 ± 1.20b | 214.76 ± 0.28b | 74.76 ± 0.67c | |
| Fermented | Roots | 29.97 ± 0.57c | 4.39 ± 0.10c | 46.41 ± 1.38c | 110.09 ± 1.27c | 88.20 ± 0.47a |
| Shoots | 40.11 ± 0.98a | 29.95 ± 0.46a | 62.30 ± 1.20a | 222.06 ± 0.22a | 76.76 ± 0.41d | |
All values were expressed as means ± standard deviation of triplicated experiments. Different letters in same column indicate significant differences as determined by Duncan’s multiple range tests (p < 0.05). 1) GAE: mg gallic acid /g solid content of sample. 2) KPE: mg kaempferol /g solid content of sample. ABTS: 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid, FRAP: Ferric reducing antioxidant power. Different letters (a, b, c, and d) represent significant differences among samples.
2.4. Antioxidant Activity of Fermented Hydroponic Ginseng
The 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), β-carotene, and ferric reducing antioxidant power (FRAP) assays were performed in this study to determine antioxidant activity. The ABTS cation accepts a hydrogen radical or electron to change to a stable diamagnetic molecule and reduced free radical. Table 2 shows the ABTS radical scavenging activities of roots and shoots extracts in fermented and non-fermented HPG. The ABTS radical scavenging activity increased from 34.21% to 46.41% and 54.69% to 62.30% in fermented hydroponic ginseng roots and shoots, respectively. The reducing power of the HPG extracts was also increased by fermentation. The HPG FRAP assay shows an increase in the ferric ion reducing power from 89.47 to 110.09 μg Fe2+/mg sample and from 214.76 to 222.06 μg Fe2+/mg sample in HPG roots and shoots, respectively (Table 2). β-Carotene-linoleic acid assay showed the lipid oxidation inhibitory activity of samples. Inhibition of linoleic acid oxidation of HPG roots and shoots increased slightly (by 102.7–104.1%) after fermentation. The different results of three methods may be due to different principles of antioxidant assay methods and, in addition, different contents of ginsenosides as well as polyphenols such as chlorogenic acid, gentisic acid, and coumaric acid according to part of ginseng plant [17]. Overall, the antioxidant activities of HPG shoots were greater than those of roots, and the antioxidant activities increased even after fermentation. Lee et al. [18] reported that the radical scavenging activities of ginseng leaves and stems were not affected by fermentation, but there were significant increases in phenolic compounds, which is contrary to our observations in this study. As TPC and TFC increased during fermentation, the antioxidant activity of fermented samples increased. It is considered that these results may occur due to the increase in low molecular weight polyphenol compounds and enzymatically bioconverted compounds produced by fermentation of L. mesenteroides KCCM 12010P.
2.5. Anti-Inflammatory Effect of Non-fermented and Fermented Hydroponic Ginseng
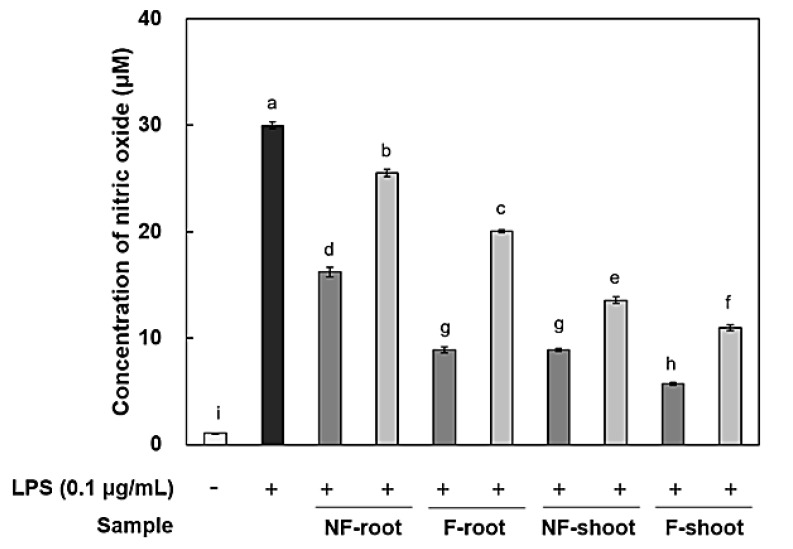
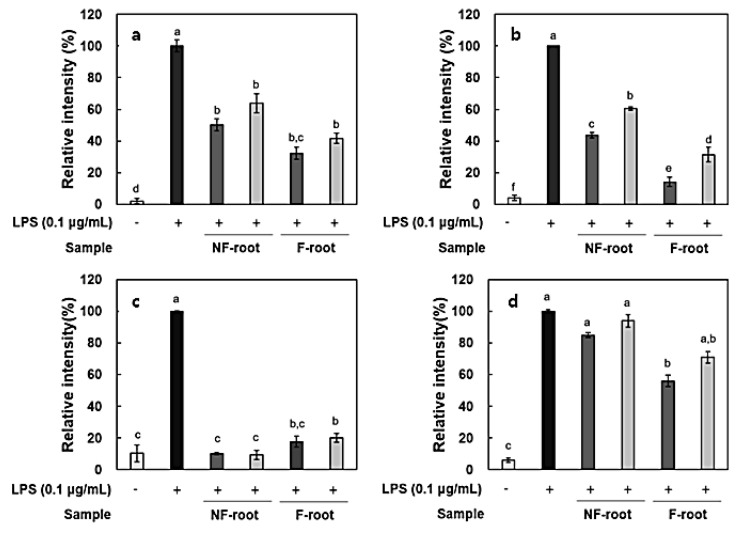
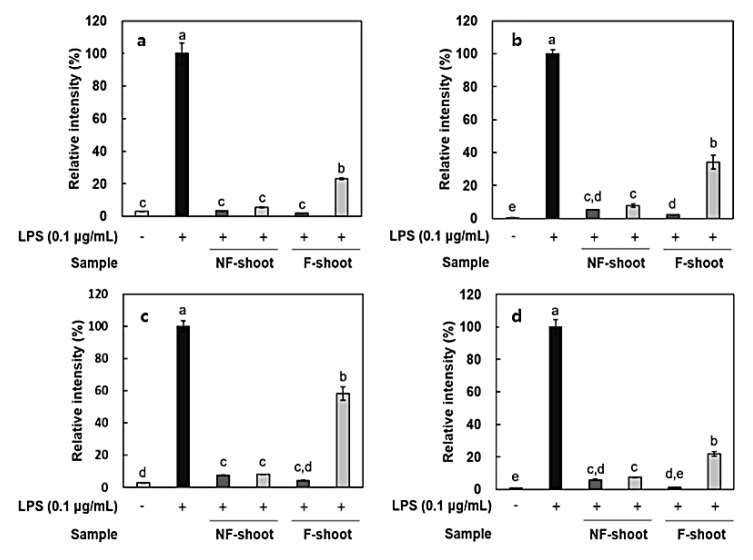
In this study, the amount of nitric oxide (NO) produced from the RAW 264.7 cells, as a form of NO2- present in the cell medium, was measured and RT-PCR was performed to measure the expression of iNOS and cytokines, including IL-6, IL-1β, and TNF-α. It has been reported that roots and shoots of hydroponic ginseng, as well as ginseng, exhibit the inhibitory effects of inflammation in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells [2]. To evaluate the anti-inflammatory activity of non-fermented and fermented HPG, cytotoxicity in RAW 264.7 cells was preliminarily assessed using a 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium (MTT) assay. No cytotoxicity was observed in samples at treatment concentrations, i.e., 100–200 μg/mL (data not shown), but there was a significant decrease in NO production after HPG fermentation (Figure 2). NO concentration in roots and shoots decreased by 54.83% and 35.73% at 200 μg/mL of fermented HPG, respectively, compared to non-fermented samples. LPS in the extracellular membrane of gram-negative bacteria has been known to increase proinflammatory cytokines, such as TNF-α, IL-6, and interleukin-1β (IL-1β), which are transcribed by NF-κB [19]. In a previous study [5], L. mesenteroides KCCM 12010P degraded ginsenoside Rb1 to Rd, compound K, and Rh2 in a stepwise manner. It seems that the amount of Rd, compound K and Rh2 in samples may increase due to bioconversion since HPG is rich in Rb1 and Rd. According to the study of Cho et al. [20], ginsenoside Rb1 inhibits TNF-α expression by suppression of NF-κB activation. The increased ginsenoside Rd, which has a suppressive effect on inflammatory responses in later stages, after ischemia, by inhibiting iNOS and COX-2 [21,22], is considered as a factor that helps to reduce NO generation (Figure 3a and Figure 4a). After fermentation, TNF-α and IL-1β expression was suppressed in the root extract treated group. In the shoot extract treated group, cytokine expression was not significantly decreased, compared with levels prior to fermentation, but the inhibition rate remained at a low level (Figure 4). As shown Figure 4 and Figure 5, it appeared that a treatment with fermented ginseng root increased anti-inflammatory effects while, in case of shoots, fermentation decreased the anti-inflammatory effect. In the HPG plant, there are many kinds of bio-active phytochemicals and each composition may be different in between root and shoot. During fermentation, some compounds can be bioconversed or the contents of each compound can be changed [5.6]. Therefore, although the compounds have the anti-inflammatory effects, the degree can be different. The major compounds for anti-inflammation in fermented HPG are still unknown.
Figure 2.
Anti-inflammatory effect in RAW 264.7 cells treated with hydroponic ginseng fermented with Leuconostoc mesenteroides KCCM 12010P. 200 μg/mL; 100 μg/mL. Values are means ± standard deviation of triplicate experiments. Different letters indicate significant differences as determined by Duncan’s multiple range tests (p < 0.05).
Figure 3.
Relative expression of inflammatory mediators in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells treated with hydroponic ginseng root. (a), Inducible nitric oxide synthase (iNOS); (b), tumor necrosis factor-α (TNF-α); (c), interleukin-6 (IL-6); and (d), interleukin-1β (IL-1β). 200 μg/mL; 100 μg/mL. NF, non-fermented; F, fermented. Values are means ± standard deviation of triplicate experiments. Different letters in same column indicate significant differences as determined by Duncan’s multiple range tests (p < 0.05). Different letters (a, b, c, and d) represent significant differences among samples.
Figure 4.
Relative expression of inflammatory mediators in LPS-stimulated RAW 264.7 cells treated with hydroponic ginseng shoot. (a), iNOS; (b), TNF-α; (c), IL-6; and (d), IL-1β. 200 μg/mL; 100 μg/mL. NF, non-fermented; F, fermented. Values are means ± standard deviation of triplicate experiments. Different letters in same column indicate significant differences as determined by Duncan’s multiple range tests (p < 0.05). Different letters (a, b, c, and d) represent significant differences among samples.
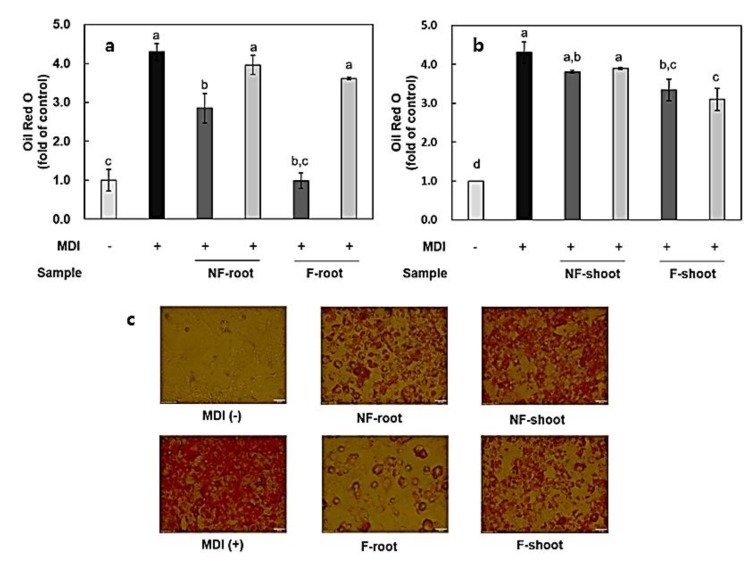
Figure 5.
Effects of fermented hydroponic ginseng root (a) and shoot (b) on lipid accumulation in differentiated 3T3-L1 cells, and Oil Red O (ORO) stained cells after differentiation (c). 200 μg/mL; 100 μg/mL. NF, non-fermented; F, fermented; MDI, insulin + dexamethasone + 3-isobutyl-1-methylxanthine (IBMX). Values are means ± standard deviation of triplicate experiments. Different letters indicate significant differences as determined by Duncan’s multiple range tests (p < 0.05). Different letters (a, b, c, and d) represent significant differences among samples.
From the macroscopic point of view, it seems that the prevention of diabetes, cancer, and cardiovascular diseases caused by chronic inflammation may be possible through the inhibitory mechanisms against such inflammation in fermented HPG.
2.6. Anti-Adipogenic Activity of Non-fermented and Fermented Hydroponic Ginseng
The effect of non-fermented and fermented HPG on lipid accumulation during adipogenesis in 3T3-L1 cells was measured by Oil Red O (ORO) staining. Each group was treated with 100–200 μg/mL of non-fermented and fermented HPG root, shoot extract. As described in previous studies, lipid droplets appeared on the second day of differentiation and increased until day nine (data not shown) [23]. ORO staining showed that the differentiation induced by MID, a mixture of insulin + dexamethasone + 3-isobutyl-1-methylxanthine (IBMX), occurred successfully (Figure 5). In the case of a group treated with fermented root, it appeared that fermentation with L. mesenteroides KCCM 12010P significantly decreased lipid accumulation during the nine-day incubation. Meanwhile, the cell viability of samples was measured via the MTT assay and it was shown that the samples (100–200 μg/mL) did not affect the viability of 3T3-L1 cells (data not shown). From these data, it appeared that lipid accumulation was inhibited by approximately 66% of the differentiated group when treated with 200 μg/mL of root extract before fermentation (Figure 5). Chae et al. [8] reported that several cytokines, such as TNF-α, IL-6, and NF-κB, involved in immune response are also secreted by adipocytes and regulate food intake, energy expenditure, and hormone functions. Therefore, the inhibition of lipid accumulation by fermented HPG roots is closely related to the inhibitory effects of these cytokines (Figure 3 and Figure 4). Hence, HPG shoots with high inflammatory cytokine inhibition rates are expected to have higher inhibitory effects on lipid accumulation in 3T3-L1 cells, but the results obtained here contradict this hypothesis. Although 200 μg/mL fermented shoot extract significantly decreased lipid accumulation by 76.08% of the differentiated control group, it has a lower inhibition rate compared to the root extract-treated group. The carbohydrate content in root and leaves are 59% and 73%, respectively [24,25], and a high sugar concentration is considered to affect lipid accumulation. In one study [26], the supplementation of a high-fat diet with hydroponic ginseng vinegar, containing ginsenoside Rg2, resulted in decreased body weight, fat weight, and liver weight in mice.
3. Materials and Methods
3.1. Materials
Two-year-old whole hydroponic ginseng (HPG) were purchased from Chungjung–Saessacksam Company, Gwangju, Republic of Korea. RAW 264.7 cells were purchased from Korean Cell Line Bank (Seoul, Korea) and 3T3-L1 cells were taken from American Type Culture Collection (ATCC), Rockville, USA. The media, Man Rogosa Sharpe (MRS) broth was purchased from Difco Laboratories, Detroit, MI, USA. The reagents, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium (MTT), insulin, dexamethasone, lipopolysaccharide (LPS), 3-isobutyl-1-methylxanthine (IBMX), and Oil Red O (ORO) were purchased from Sigma-Aldrich Co., St. Louis, MO, USA. Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin solution, and fetal bovine serum (FBS) were purchased from HycloneTM (Logan, UT, USA).
3.2. Microorganisms
L. mesenteroides KCCM 12010P was screened and isolated from white cabbage kimchi and identified by the Korean Culture Center of Microorganisms (KCCM, Seoul, Korea). The strain was cultured in MRS broth at 37 °C for 24 h before inoculation into HPG samples.
3.3. Determination of Bacterial Production of Enzymes
Enzyme produced by L. mesenteroides KCCM 12010P was determined using API ZYM kit (Biomerieux Inc., Marcy l’Etoile, France) according to the company’s manual. A 75 μL of 107 CFU/mL of the strain in phosphate buffered saline (PSB) was inoculated into each cupule at 37 °C for 4 h. A sample of empty cupule was used as a control. ZYM-A and ZYM-B reagents were then mixed and a color change of the solutions was observed. Enzyme activities during culturing were expressed as 0, 5, 10, 20, or 40 nM, as indicated in the API ZYM kit.
3.4. Preparation for Hydroponic Ginseng Fermentation
Two-year-old HPG was separated into roots and shoots. Each part was lyophilized and extracted twice with 70% methanol in a reflux system. The extracts were concentrated using a vacuum evaporator (EYELA N-1000V, Tokyo, Japan) and then freeze-dried. pH of lyophilized extracts in distilled water at 5% (w/v) was adjusted to 6.5 to induce an optimum growth rate. The samples were autoclaved at 121 °C for 15 min and L. mesenteroides KCCM 12010P was inoculated into 5% broth of hydroponic ginseng roots and shoots. The initial number of cells was adjusted to approximately 6 Log CFU/mL. The non-fermented and fermented extracts were filtered with a 0.45 μm membrane filter and then used as samples during subsequent experiments.
3.5. Changes in Viable Number of Cells and pH during Fermentation
During fermentation, samples were taken from the cultures at 0, 3, 6, 9, 12, and 24 h to estimate the change in viable numbers of cells and pH. The bacterial count for L. mesenteroides KCCM 12010P on MRS agar was estimated using the plate count method after incubation at 37 °C for 1 d. The changes in pH were determined using a pH meter (WTW-720, Weilheim, Germany).
3.6. Measurement of Total Flavonoid Content
The total flavonoid content (TFC) was analyzed using the aluminum chloride assay described by Eom et al. [15]. The samples concentration was 5.0 mg/mL. TFC was calculated in milligrams of kaempferol equivalents (KPE) per g solid sample. The kaempferol calibration curve equation was as follows:
| Y = 0.0068X + 0.0426, R2 = 1 | (1) |
where X is the amount of kaempferol content equivalent to gallic acid, Y is the absorbance at 415 nm.
3.7. Measurement of Total Polyphenol Content
The total polyphenol content (TPC) was measured using a slightly modified Folin–Ciocalteu method of Eom et al. [15]. The hydroponic ginseng shoot and root extracts were prepared in 5.0 mg/mL aliquots. Gallic acid was used as a standard and TPC concentration was expressed as milligrams of gallic acid equivalents (GAE) per g solid sample. The gallic acid calibration curve equation was as follows:
| Y = 0.0019X-0.0114, R2=0.9979 | (2) |
where X is the amount of total flavonoid content equivalent to kaempferol, Y is the absorbance at 750 nm.
3.8. 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) Radical Scavenging Assay
An ABTS radical scavenging assay was performed as described by Chung et al. [17], with subtle modifications. A radical cation solution of ABTS was prepared with a 1:1 mixture of 14 mM ABTS and 5 mM potassium persulfate (in 0.1 M potassium phosphate buffer pH 7.4). The mixture was incubated at 25 °C for 16 h. The mixture was then diluted with 0.1 M potassium phosphate buffer till the absorbance was 0.7 (0.7 ± 0.02) at a wavelength of 734 nm. The antioxidant rate was calculated as follows:
| (3) |
where Ac and As are the absorbance for a control (distilled water) and sample, respectively.
3.9. Ferric Reducing Antioxidant Power (FRAP) Assay
The ferric ion reducing effect of samples were determined via a modified FRAP assay method [27]. The cocktail solution was made by mixing acetate buffer (300 mM, pH 3.6), 10 mM 2,4,6-tris (2′-pyridyl)-s-triazine in 40 mM HCl, and 20 mM ferric chloride hexahydrate solution at a ratio of 10:1:1 in a 37 °C water bath. The sample (50 μL) was added to 950 μL cocktail solution and incubated under a dark condition at 25 °C for 30 min. Fe3+ to Fe2+ reduction was monitored by measuring the absorbance at 593 nm. Ferrous sulfate heptahydrate (FeSO4 ·7H2O) was used as a standard reagent.
3.10. β-Carotene-linoleic Acid Assay
A β-carotene-linoleic acid assay was used to determine the inhibitory effect on lipid oxidation of samples. The procedure was carried out using a slightly modified method of Eom et al. [5]. β-carotene (2 mg), linoleic acid (44 μL), and Tween 80 (200 μL) were mixed thoroughly in 10 mL chloroform. The mixture was evaporated with a rotary evaporator (EYELA N-1000V, Tokyo, Japan) at 42 °C. The residue was dissolved in 100 mL of distilled water and mixed to form an emulsion. The absorbance of this sample was controlled to 1.8–2.0 at 470 nm. A 500 μL sample was added to 4.5 mL aliquots of the emulsion. After reaction at 50 °C for 3 h, the absorbance was measured at 470 nm. The inhibition rate of β-carotene-linoleic acid oxidation was determined using the following equation:
| (4) |
where A0 and At is the absorbance value at the start of incubation for sample and the absorbance after 3 h of reaction, respectively.
3.11. Measurement of Nitric Oxide (NO) Production in RAW 264.7 Macrophage Cells
Analysis of NO produced in RAW 264.7 cell was carried out using the method of Cha et al. [28]. The RAW 264.7 cells were seeded in a microplate. The cell numbers were adjusted to 2 × 105 cells/well. After incubation for 2 h at 37 °C, the cells were treated with various concentrations of samples and stimulated with 0.1 μg/mL of LPS for 24 h. Each medium was transferred to another microplate and the nitrite concentration was determined by adding 100 μL of Greiss reagent. The absorbencies were detected at 570 nm with an ELISA reader (Emax, Torrance, CA, USA). The amount of nitrite present was calculated from a sodium nitrite (NaNO2) standard curve.
3.12. Measurement of Expression of Inflammation-Related Mediators
Total mRNA from RAW 264.7 cells was extracted using a RNeasy® Mini Kit, QIAGEN, Hilden, Germany. The cDNA was synthesized by a RevertAid First Strand cDNA Synthesis Kit, Thermo Fisher Scientific, UK, followed by a reverse transcription-polymerase chain reaction (RT-PCR), using a SensiFAST™ SYBR No-ROX Kit (Bioline, London, UK). The primers used in this study are presented in Table 3.
Table 3.
Primer sequence of genes used for a reverse transcription-polymerase chain reaction.
| Forward | Reverse | |
|---|---|---|
| β-actin | 5′-GTCGGCCTAGGCACCAG-3′ | 5′-GGAGGAAGAGGATGCGGCAGT-3′ |
| iNOS | 5′-CCCTTCCGAACTTTCTGGCAGCAGC-3′ | 5′-GGCTGTCAGAGTCTCGTGGCTTTGG-3′ |
| TNF-α | 5′-GCAGAAGAGGCACTCCCCCA-3′ | 5′-GATCCATGCCGTTGGCCAGG-3′ |
| IL-1β | 5′-CAGGATGAGGACATGAGCACC-3′ | 5′-CTCTGCAGACTCAAACTCCAC-3′ |
| IL-6 | 5′-AGTTGCCTTCTTGGGACTGA-3′ | 5′-TTCTGCAAGTGCATCGT-3′ |
iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1beta; IL-6, interleukin-6.
3.13. Cell Viability Assay
Cell viability was assayed through MTT assay using the 3T3-L1 preadipocyte [29]. The cells were inoculated in a 96-well microplate at a concentration of 1 × 105 cells per well, and incubated for 1 d at 37 °C. The cells were treated with 100 μL fermented HPG samples. After 44 h of incubation, the media in the wells were discarded. MTT solution (50 μL) was added and incubated for 4 h, after which 50 μL dimethyl sulfoxide (DMSO) was also added per well to dissolve the crystals. Absorbance was measured at 570 nm using an ELISA reader (Emax, Torrance, CA, USA).
3.14. Cell Culture and Pre-Adipocyte Differentiation
The 3T3-L1 cell line was maintained and differentiated using modified methods of Nam et al. [30]. They were seeded in a 12-well plate (2 × 105 cells/well) in DMEM with 1% penicillin-streptomycin solution and 10% FBS. Prior adipocyte differentiation induction, 3T3-L1 cells were grown to post-confluence, and differentiation was induced by DMEM mixture, containing 10% FBS, 115 μg/mL IBMX in 0.35 M KOH, 10 μM dexamethasone in ethanol (day 0), 10 μg/mL insulin in 0.1 N filtered HCl, and different sample concentrations. After 3 days (day 3), the culture medium was replaced with other DMEM mixture, containing 10% FBS and 10 μg/mL insulin. After another 3 days (day 6), the medium was replaced with DMEM mixture, only containing 10% FBS. By day 9, the cells were fully differentiated and were used for Oil Red O staining.
3.15. Oil Red O Staining
On the ninth day of differentiation above, accumulated lipids in adipocyte cells were stained using the modified method described by Nam et al. [30] to detect the rate of adipogenesis. Cells were washed mildly using phosphate buffered saline (PBS) and fixed with 1 mL 10% formalin for 1 h in the dark. After washing with formalin, fixed cells were thoroughly dried in a hood. One milliliter of ORO working solution (ORO stock solution: Water = 6:4) was treated for 20 min. Then, cells were washed using distilled water and observed with an Olympus IX51 inverted microscope with Camedia C-4040 camera (Center Vally, PA, USA). Isopropanol (500 μL) was added to elute stained lipids from cells and the absorbance was measured at 504 nm.
3.16. Statistical Analysis
All experiments were repeated three times. The values were presented as mean ± standard deviation (SD). Using these data, one-way analysis of variance (ANOVA) was performed using SPSS software (version 20, SPSS Inc., Chicago, IL, USA). A Duncan’s multiple range test was used to test for significant differences between treatments at p < 0.05.
4. Conclusions
This study aimed to evaluate the antioxidant, anti-inflammatory, and anti-adipogenic activities of hydroponic ginseng root and shoot fermented by lactic acid bacteria. During 24 h fermentation in 5% broth of HPG-root and HPG-shoot extract, L. mesenteroides KCCM 12010P growth was not affected significantly. Furthermore, HPG roots and shoots fermented by L. mesenteroides KCCM 12010P showed significant increments in total polyphenolic compounds and flavonoid contents due to bioconversion driven by enzymes, including β-glucosidase. Antioxidant activity was enhanced in both fermented samples, resulting in increased phenolic compound content. Fermented HPG suppressed the production of both iNOS and NO and the expression of inflammation-related cytokines, including TNF-α, IL-1β, and IL-6. Additionally, lipid accumulation was suppressed in 3T3-L1 cells when the fermented samples were treated. Meanwhile, Hsu et al. [31] reported that phenolic acids and flavonoids reduced intracellular triglyceride content by elucidating GPDH activity, which is involved in adipogenesis. From these results, L. mesenteroides KCCM 12010P improved HPG biofunctional activities, such as antioxidant, anti-inflammatory, and antiadipogenic effects, indicating that fermented HPG could be applied in the functional food or medicinal industry as a potent bioactive material for human health.
Author Contributions
Conceptualization, H.-D.P.; methodology, K.-T.K.; software, J.E.H.; validation, K.-T.K.; formal analysis, J.E.H.; investigation, H.-D.P.; resources, J.E.H.; data curation, J.E.H.; writing—original draft preparation, J.E.H.; writing—review and editing, K.-T.K.; visualization, K.-T.K.; supervision, H.-D.P.; project administration, H.-D.P.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Kim J., Byeon H., Im K., Min H. Effects of ginsenosides on regulatory T cell differentiation. Food Sci. Biotechnol. 2018;27:227–232. doi: 10.1007/s10068-017-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang J.E., Suh D.H., Kim K.T., Paik H.D. Comparative study on anti-oxidative and anti-inflammatory properties of hydroponic ginseng and soil-cultured ginseng. Food Sci. Biotechnol. 2019;28:215–224. doi: 10.1007/s10068-018-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garruti D.S., Franco M.R.B., da Silva M.A.A.P., Janzantti N.S., Alves G.L. Assessment of aroma impact compounds in a cashew apple-based alcoholic beverage by GC–MS and GC-olfactometry. LWT- Food Sci. Technol. 2006;39:373–378. doi: 10.1016/j.lwt.2005.02.006. [DOI] [Google Scholar]
- 4.Jung J., Jang H.J., Eom S.J., Choi N.S., Lee N.K., Paik H.D. Fermentation of red ginseng extract by the probiotic Lactobacillus plantarum KCCM 11613P: ginsenoside conversion and antioxidant effects. J. Ginseng Res. 2019;43:20–26. doi: 10.1016/j.jgr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eom S.J., Hwang J.E., Kim H.S., Kim K.-T., Paik H.-D. Anti-inflammatory and cytotoxic effects of ginseng extract bioconverted by Leuconostoc mesenteroides KCCM 12010P isolated from kimchi. Int. J. Food Sci. Technol. 2018;53:1331–1337. doi: 10.1111/ijfs.13713. [DOI] [Google Scholar]
- 6.Gan R.Y., Shah N.P., Wang M.F., Lui W.Y., Corke H. Lactobacillus plantarum WCFS1 fermentation differentially affects antioxidant capacity and polyphenol content in mung bean (Vigna radiata) and soya bean (Glycine max) milks. J. Food Process Preserv. 2017;41:e12944. doi: 10.1111/jfpp.12944. [DOI] [Google Scholar]
- 7.Reyes-Gordillo K., Shat R., Muriel P. Oxidative stress and inflammation in hepatic diseases: current and future therapy. Oxid. Med. Cell Longev. 2017 doi: 10.1155/2017/3140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim M.O., Jang J.H., Lee H.E., Jung H.K., Cho H.W. Antioxidant effects of Geranium nepalense ethanol extract on H2O2-induced cytotoxicity in H9c2, SH-SY5Y, BEAS-2B, and HEK293. Food Sci. Biotechnol. 2017;26:1045–1053. doi: 10.1007/s10068-017-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chae G.N., Kwank S.J. NF-κB is involved in the TNF-α induced inhibition of the differentiation of 3T3-L1 cells by reducing PPARγ expression. Exp. Mol. Med. 2003;35:431–437. doi: 10.1038/emm.2003.56. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales-González Á., Esquivel-Chirino C., Durante-Montiel I., Sánchez-Rivera G., Valadez-Vega C., Morales-González J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y., Oh G.H., Kim M.B., Hwang J.K. Fucosterol inhibits adipogenesis through the activation of AMPK and Wnt/beta-catenin signaling pathways. Food Sci. Biotechnol. 2017;26:821–827. doi: 10.1007/s10068-017-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller D.E. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 2000;11:212–217. doi: 10.1016/S1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 13.Seo J.Y., Lee J.H., Kim N.W., Kim Y.J., Chan S.H., Ko N.Y., Her E., Yoo Y.H., Kim J.W., Lee B.Y., et al. Inhibitory effects of a fermented ginseng extract, BST204, on the expression of inducible nitric oxide synthase and nitric oxide production in lipopolysaccharide-activated murine macrophages. J. Pharm. Pharmacol. 2005;57:911–918. doi: 10.1211/0022357056497. [DOI] [PubMed] [Google Scholar]
- 14.Jeon W.J., Oh J.S., Park M.S., Ji G.E. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytotherapy Res. 2013;27:166–172. doi: 10.1002/ptr.4706. [DOI] [PubMed] [Google Scholar]
- 15.Eom S.J., Hwang J.E., Kim K.-T., Paik H.-D. Increased antioxidative and nitric oxide scavenging activity of ginseng marc fermented by Pediococcus acidilactici KCCM11614P. Food Sci. Biotechnol. 2017;27:185–191. doi: 10.1007/s10068-017-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez H., Curiel J.A., Landete J.M., de las Rivas B., Felipe F.L., Gómez-Cordovés C., Mancheño J.M., Muñoz R. Food phenolics and lactic bacteria. Int. J. Food Microbiol. 2009;132:79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Chung I.M., Lim J.J., Ahn M.S., Jeong H.N., An T.J., Kim S.H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax Ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016;40:68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K.S., Seong B.J., Kim G.H., Kim S.I., Han S.H., Kim H.H., Baik N.D. Ginsenoside, phenolic acid composition and physiological significances of fermented ginseng leaf. J. Korean Soc. Food Sci. Nutr. 2010;39:1194–1200. [Google Scholar]
- 19.Young R., Bush S.J., Lefevre L., McCulloch M.E.B., Lisowski Z.M., Muriuki C., Waddell L.A., Sauter K.A., Pridans C., Clark E.L., et al. Species-specific transcriptional regulation of genes involved in nitric oxide production and arginine metabolism in macrophages. ImmunoHorizons. 2018;2:27–37. doi: 10.4049/immunohorizons.1700073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho J.Y., Yoo E.S., Baik K.U., Park M.H., Han B.H. In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor (TNF)-α production and its modulation by known TNF-α antagonists. Planta Med. 2001;67:213–218. doi: 10.1055/s-2001-12005. [DOI] [PubMed] [Google Scholar]
- 21.Ye R., Yang Q., Kong X., Han J., Zhang X., Zhang Y., Li P., Liu J., Shi M., Xiong L., et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem. Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang J.W., Do H.J., Kim O.Y., Chung J.H., Lee J.Y., Park Y.S., Hwang K.Y., Seong S.I., Shin M.J. Fermented soy bean extract suppresses differentiation of 3T3-L1 preadipocytes and facilitates its glucose utilization. J. Funct. Food. 2015;15:516–524. doi: 10.1016/j.jff.2015.04.002. [DOI] [Google Scholar]
- 24.Song B.H., Chan Y.G., Lee K.A., Lee S.W., Kang S.W., Cha S.W. Studies on analysis of growth characteristics, ability of dry matter production, and yield of Panax ginseng C. A. Meyer at different growth stages with different cultivars and shading nets in paddy field. Korean J. Medicin Crop. Sci. 2011;19:90–96. doi: 10.7783/KJMCS.2011.19.2.090. [DOI] [Google Scholar]
- 25.Jang J.G., Lee K.S., Kwon D.W., Oh H.K. Chemical compositions of Korean ginseng with special reference to the part of ginseng plant. J. Ginseng Res. 1987;6:84–89. [Google Scholar]
- 26.Oh Y.J., Kwon S.H., Choi K.B., Kim T.S., Yeo I.H. Effect of vinegar made with hydroponic-cultured Panax ginseng C. A. Meyer on body weight and lipid metabolism in high-fat diet-fed mice. Korean Food Sci. Biotechnol. 2014;46:743–749. doi: 10.9721/KJFST.2014.46.6.743. [DOI] [Google Scholar]
- 27.Gülçin İ., Huyut Z., Elmastaş M., Aboul-Enein H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010;3:43–53. doi: 10.1016/j.arabjc.2009.12.008. [DOI] [Google Scholar]
- 28.Cha B.J., Park J.H., Shrestha S., Baek N.I., Lee S.M., Lee T.H., Kim J., Kim G.S., Kim S.Y., Lee D.Y. Glycosyl glycerides from hydroponic Panax ginseng inhibited NO production in lipopolysaccharide-stimulated RAW264.7 cells. J. Ginseng Res. 2015;39:162–168. doi: 10.1016/j.jgr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Wang J., Liu M., Zhao H., Yaqoob S., Zheng M., Cai D., Liu J. Antiobesity effectsof ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients. 2018;10:830. doi: 10.3390/nu10070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nam H., Jung H., Kim Y., Kim B., Kim K.H., Park S.J., Suh J.G. Aged black garlic extract regulates lipid metabolism by inhibiting lipogenesis and promoting lipolysis in mature 3T3-L1 adipocytes. Food Sci. Biotechnol. 2018;27:575–579. doi: 10.1007/s10068-017-0268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu C.L., Yen G.C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007;55:8404–8410. doi: 10.1021/jf071695r. [DOI] [PubMed] [Google Scholar]