Figure 2.
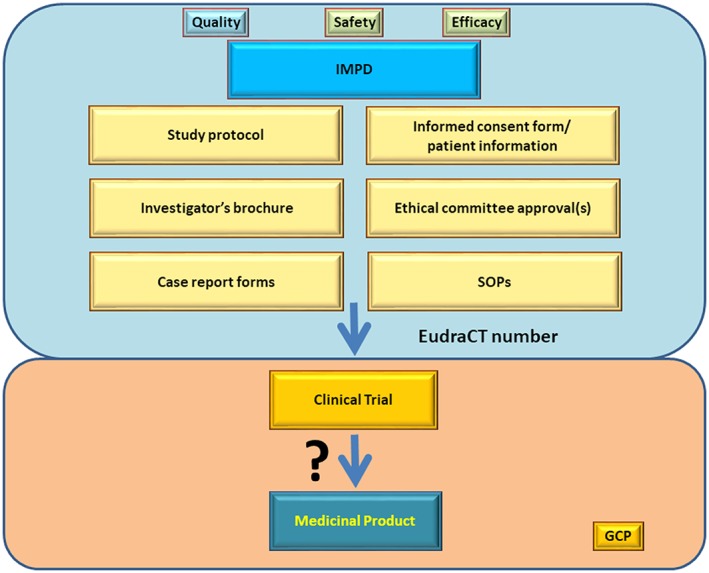
Submission process scheme. Blue panel. Investigational medicinal product dossier (IMPD) containing all information obtained in the upper three panels of Figure 1 (quality aspects of radionuclide, ligand and final radiopharmaceutical formulation production/preparation; safety and efficacy aspects from preclinical animal studies) together with some other key documents like study protocol, investigator's brochure, or informed consent forms to various SOPs enables submission of the trial under designated EudraCT number. Orange panel. After approval, the clinical trial can be initiated and could potentially lead to a medicinal product (radiopharmaceutical) with a marketing authorization
