Abstract
Purpose
To systematically review the evidence for health coaching as an intervention to improve health‐related quality of life (HRQoL) and reduce hospital admissions in people with chronic obstructive pulmonary disease (COPD).
Methods
We systematically searched MEDLINE, EMBASE, PsycINFO, and CINAHL from database inception to August 2018 to identify all randomized controlled trials (RCTs) of health coaching for people with COPD. Eligible health coaching interventions had to include three components: goal setting, motivational interviewing, and COPD‐related health education. Data were extracted on study characteristics and the effects of the intervention on HRQoL, hospital admissions, physical activity, self‐care behaviour, and mood. Study quality was appraised by two authors using the Cochrane tool for assessing the risk of bias in RCTs. Effect sizes (standardized mean differences [SMD] or odds ratios [OR]) with 95% confidence intervals (CIs) were calculated and pooled using random effects meta‐analyses.
Results
Of 1578 articles, 10 RCTs were included. Meta‐analysis showed that health coaching has a significant positive effect on HRQoL (SMD = −0.69, 95% CI: −1.28, −0.09, p = .02, from k = 4) and leads to a significant reduction in COPD‐related hospital admissions (OR = 0.46, 95% CI: 0.31, 0.69, p = .0001, from k = 5), but not in all‐cause hospital admissions (OR = 0.70, 95% CI: 0.41–1.12, p = .20, from k = 3). Three of four studies reported significant improvements to self‐care behaviours such as medication adherence and exercise compliance.
Conclusions
This is the first systematic review to show that health coaching may be a candidate intervention to improve HRQoL and reduce costly hospital admissions in people with COPD.
Statement of contribution.
What is already known on this subject?
COPD is a leading cause of death worldwide and considerably reduces HRQoL. In turn, HRQoL is associated with a range of adverse health outcomes in COPD.
Health coaching is a self‐management intervention for people with long‐term conditions such as COPD. Studies have examined whether health coaching improves HRQOL and other health outcomes in people with COPD, but no systematic review has been conducted.
What does this study add?
The first systematic review and meta‐analysis of RCTs of health coaching for people with COPD.
Health coaching may be a candidate intervention for improving HRQoL and reducing COPD‐related hospital admissions in people with COPD.
The need to establish the most effective health coaching components, delivery modality, and economic impact.
Keywords: chronic obstructive pulmonary disease, health coaching, health‐related quality of life, hospital admissions, HRQoL, self‐management intervention
Background
Chronic obstructive pulmonary disease (COPD) is a long‐term condition (LTC) that affects approximately 300 million people globally (Vos et al., 2015). It is currently the fifth leading cause of death worldwide and is expected to become the third by 2030 (World Health Organization, 2016). In the United Kingdom (UK), COPD exacerbations are the second most common cause of emergency hospital admissions and COPD is one of the most costly inpatient conditions to be treated by the National Health Service (NHS; Department of Health, 2012). Similarly, in the United States of America (USA), almost 700,000 hospitalizations each year are due to COPD (Almagro et al., 2006). These hospitalizations account for a substantial proportion of the annual direct medical costs of COPD, yet they are potentially preventable (Roberts et al., 2002). As the global population increases and ages, the human and economic costs of COPD will continue to increase.
COPD is a progressively disabling condition and is associated with impaired health‐related quality of life (HRQoL; Garrido et al., 2006; Jones et al., 2011). HRQoL is a measure of the impact of disease on daily life and subjective well‐being (Jones, 1991). It is a multidimensional concept that includes domains related to the physical, social, and psychological impact of illness (Bakas et al., 2012). As COPD is not reversible and lung function will not improve, HRQoL is a key, modifiable patient‐centred factor that is associated with important health outcomes for people.
Poor HRQoL in COPD is negatively associated with symptom severity (Garrido et al., 2006; Hu & Meek, 2005), reduced physical functioning (Hu & Meek, 2005), depression, and anxiety (Blakemore et al., 2014), and an increased risk of exacerbations (Seemungal et al., 1998) and mortality (Domingo‐Salvany et al., 2002). Furthermore, people with moderate to severe COPD and poor HRQoL are at a greater risk of readmission to hospital and are more likely to be issued a home nebulizer and to be referred to a respiratory specialist (Osman, Godden, Friend, Legge, & Douglas, 1997). In addition, clinical measures of pulmonary function (i.e., FEV1 and FVC) have been found to be unrelated to readmission in people with low HRQoL. In a systematic review of prospective longitudinal cohort studies, it was found that depression predicted poor HRQoL in people with COPD (Blakemore et al., 2014). Depression in COPD patients has also been found to be associated with an increased risk of hospital admission (Dickens et al., 2012; Guthrie et al. 2016). Thus, it is plausible that differences in HRQoL can better explain the variance in use of health care resources than differences in pulmonary function. Improving HRQoL in people with COPD may be a promising route through which to reduce associated urgent health care costs.
Self‐management interventions can improve HRQoL and reduce exacerbations in people with COPD (Cannon et al., 2016; da Silva, 2011; Peytremann‐Bridevaux, Staeger, Bridevaux, Ghali, & Burnand, 2008). Such interventions focus on increasing patients’ knowledge of COPD and improving their confidence and skills in managing symptoms and treatment. Intervention content typically includes action planning, health education, and support from a health care professional (Barlow, Wright, Sheasby, Turner, & Hainsworth, 2002; Monninkhof et al., 2003). The aim is to facilitate behaviour change through a combination of health education and promotion of healthy behaviours (e.g., smoking cessation). The King's Fund (2012) has highlighted the importance of patient‐centred, tailored care plans and has called for greater efforts to enable patients to co‐design a personalized self‐management plan through interventions such as health coaching.
Health coaching is rapidly emerging as an approach to improve patient self‐management and facilitate healthy behaviour change in LTCs. It is a patient‐centred intervention that aims to improve disease self‐management by motivating patients to achieve goals that improve their HRQoL and overall health. It is believed that the distinct ‘coaching’ element, aided by the use of motivational interviewing techniques, is key to empowering people to improve and maintain a good health status (Linder, Menzies, Kelly, Taylor, & Shearer, 2003). The potential of health coaching for improving a range of patient outcomes has been demonstrated across different populations with LTCs, such as type 2 diabetes mellitus, congestive heart failure, and rheumatoid arthritis (Kivelä, Elo, Kyngäs, & Kääriäinen, 2014). In their systematic review, Kivelä et al. (2014) found that health coaching improved various physiological (e.g., weight loss and HbA1c), behavioural (e.g., physical activity), psychological (e.g., self‐efficacy and mental health status), and social (e.g., social support) outcomes across 11 of 13 studies.
To date, there have been several studies of health coaching for people with COPD, but results have been inconclusive as to any benefits on HRQoL, health care utilization, physical activity, and other health outcomes. However, qualitative process evaluations have indicated high acceptability of this intervention for people (Walters et al., 2012). It is possible that the inconsistent findings across studies are partly due to the lack of consensus regarding the optimal components and content of an effective health coaching intervention (Wolever et al., 2013). Researchers have adopted varying definitions of health coaching, resulting in different delivery modalities and a range of intervention strategies targeted for psychological and behavioural change. In an attempt to resolve this issue, Wolever et al. (2013) systematically reviewed the medical and health literature on health and wellness coaching to establish a consensus definition of health coaching. Based on their findings, the present review defines health coaching as a patient‐centred approach wherein the individual and coach work together to set goals to improve health outcomes, achieving these through active health education processes and motivational interviewing techniques applied by the coach. Therefore, the present review aims to systematically review the evidence for health coaching as an intervention for people with COPD. The specific objectives of the review are to:
Examine whether health coaching is an effective intervention for improving HRQoL in people with COPD.
Examine whether health coaching is an effective intervention for improving other health outcomes in people with COPD, such as hospital admissions, physical activity, self‐care adherence, and mood.
Method
This review is reported according to the PRISMA statement (Moher, Liberati, Tetzlaff, & Altman, 2009; Appendix S1). The PROSPERO protocol registration number for this systematic review is CRD42016050329 (Peters, Blakemore, & Long, 2016).
Search strategy and study inclusion process
We initially conducted a systematic search of four electronic databases (MEDLINE, EMBASE, PsycINFO, and CINAHL) from database inception to December 2016 to identify RCTs of health coaching interventions for people with COPD. The searches were then rerun in all databases in August 2018 and limited to the 2016–2018 publication period. Search strategies were developed within the team and used Medical Subject Headings (MeSH) terms, keywords, and suitable variants on COPD, health coaching, HRQoL, and hospital admissions (Appendix S2). Microsoft Excel and Endnote software were used to organize articles retrieved by the searches.
Following the database searches, duplicated articles were excluded and the first author screened the titles and/or abstracts of all identified articles and retrieved the full texts of any potentially eligible articles. The full texts were assessed against the inclusion and exclusion criteria to determine eligibility (Table 1). The PICOS (‘Population’, ‘Intervention, ‘Controls’, ‘Outcomes’, and ‘Study Design’) tool was used to formulate the eligibility criteria (Centre for Reviews and Dissemination, 2009). An inter‐rater reliability test on a subsample (20%; k = 11) of potentially eligible articles retrieved in the December 2016 searches was completed by a second author and demonstrated high agreement (91%). The single disagreement was related to the definition of motivational interviewing, and this was resolved through discussion with a third author. Sixteen per cent (k = 9) of the full texts were also screened by a second author before making a final decision. These were not randomly selected but were studies for which eligibility was more ambiguous. We requested further information from seven authors (reflecting eight articles), of whom five replied. Of these, four articles were included and the remaining four excluded (following three unanswered requests and one reply that contained details that led to the exclusion of the article).
Table 1.
Inclusion and exclusion criteria using the PICOS tool
| Eligibility criteria | |
| Population | Adults (aged 18+) with COPD diagnosed and/or confirmed by spirometry as an forced expiratory volume in 1 s (FEV1) < 80% of the predicted values according to GOLD (2017) criteria or FEV1/forced vital capacity (FVC) ratio of < 0.70. Co‐ or multi‐morbidities were included, except asthma, a current respiratory disorder other than COPD, or serious and unstable cardiovascular disease (unless separate data for COPD patients are reported) |
| Intervention |
Intervention must include evidence of goal setting, motivational interviewing techniques, and COPD‐related health education. Interventions that do not have clear evidence of all three components will be excluded The intervention must be delivered by a qualified HCP, over a minimum of two sessions, either face to face, by telephone, online, email, tablet, smartphone, or a combination of these methods. Interventions that include group, instead of individual, coaching sessions will be excluded |
| Control | Trials must consist of one group that received the health coaching intervention and one group that received either treatment as usual, wait‐list control, or a no intervention control group |
| Outcomes |
Primary outcome measure: a validated, self‐report measure of general quality of life and/or disease‐specific HRQoL Secondary outcome measure(s): COPD‐related hospital admissions (validated by hospital records). We accepted any objective measure of physical activity. Self‐care adherence and mood must be measured using a self‐report measure. Secondary outcome measures will be included as and when reported |
| Study design | Randomized controlled trials (RCTs) |
Data extraction
A data extraction sheet was developed, piloted, and refined, following data extraction guidelines (Centre for Reviews and Dissemination, 2009; Higgins & Green, 2011). One author extracted data on study setting, design, intervention content and characteristics, health professionals delivering the intervention, sample characteristics, outcome measures, and results. A second author extracted the data needed to undertake the meta‐analyses.
Data analysis and synthesis
We conducted a narrative synthesis of the results, including all primary and secondary outcome measures. Where possible, we conducted meta‐analysis in Review Manager 5.3. Meta‐analysis was deemed possible where the necessary data, as outlined below, were available in the published paper. We did not contact authors for additional data to include in the meta‐analysis. We also did not make any decisions about inclusion in the meta‐analysis based on the quality of the studies.
We calculated the effect size (standardized mean differences [SMD]) and 95% confidence intervals (CIs) for continuous outcomes where means, standard deviations (SD), and sample sizes were available for both the health coaching and control groups. SMD was chosen to allow comparison between studies that had measured the same outcome using different measures (e.g., measured HRQoL with the St George's Respiratory Questionnaire [SGRQ; Jones, Quirk, & Baveystock, 1991] and the SGRQ‐C). SMD was calculated by dividing the difference in the mean outcome between the two groups with the pooled SD. Appropriate transformations were made to ensure that the direction of each scale was the same (mean scores multiplied by −1; Borenstein, Hedges, Higgins, & Rothstein, 2009; Higgins & Green, 2011). Studies that presented change scores, or baseline and change scores, were not eligible for inclusion in the meta‐analysis, as per Cochrane guidelines which state that final value and change‐from‐baseline measures should not be combined together as SMD (Higgins & Green, 2011).
A SMD of zero would be interpreted as health coaching and treatment as usual having equivalent effects on the outcome. In the case of the SGRQ, improvement in HRQoL is associated with lower scores, and therefore, an SMD of lower than zero would indicate that health coaching is more efficacious than treatment as usual. The size of the effect can be interpreted using Cohen's (1988) guidelines: small effect (SMD = 0.2), medium effect (SMD = 0.5), and a large effect (SMD = 0.8).
Odds ratios (ORs) and 95% CIs were calculated for hospital admissions where the number of subjects admitted for COPD‐related reasons and/or all‐cause admissions and sample size was reported. The OR is the ratio of odds of hospital admissions in the intervention condition to the odds of hospital admissions in the usual care condition (Borenstein et al., 2009). An OR equal to 1 would indicate that there is no difference in effect between the intervention condition and usual care condition, whereas an odds ratio less than 1 indicates that the intervention reduced hospital admissions.
Where there were multiple follow‐up data points, SMDs and ORs were calculated for the follow‐up data collected nearest to 6 months to maximize consistency across studies. Effects of the interventions were pooled across independent studies using random effects models, weighted using the inverse of the variance (DerSimonian & Laird, 1986).
Heterogeneity across studies was assessed using the chi‐squared test (X2), which gives an assessment of whether the differences found are due to chance. The I 2 statistic was also calculated, which shows the percentage of variability in the effect estimate that is due to heterogeneity rather than chance (Higgins & Green, 2011). An I 2 of 0–40% can be interpreted as low heterogeneity, 30–60% as moderate heterogeneity, 50–90% as substantial heterogeneity, and 75–100% as considerable heterogeneity (Higgins & Green, 2011). We did not formally test for publication bias in this review because of the small number of studies eligible for inclusion (Lau, Ioannidis, Terrin, Schmid, & Olkin, 2006).
Quality assessment
Study quality was appraised using the Cochrane tool for assessing the risk of bias in RCTs (Higgins et al., 2011). This tool features six domains of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. Within each domain, judgements were made for one or more items that relate to aspects or outcomes of the domain. Assessing the risk of bias for each item involved two steps. Firstly, a summary of the trial information pertinent to the decision of bias for that item was made. Secondly, each item was assigned a rating of high, low, or unclear risk of bias. This judgement was based on the extent to which the study characteristics are deemed sufficient to have had an effect on the study results or conclusions.
Two authors independently assessed the risk of bias in the included studies (79% agreement). Any disagreements were resolved through discussion, and if a decision could not be reached, a third author was consulted. The authors rated items across the studies differently on 17 of 80 occasions and discussed these items before agreeing on a final rating. Most disagreements were because of differing opinions regarding whether the primary study authors had provided sufficient detail in order to give a rating of high, low, or unclear bias. The authors revisited Higgins et al. (2011) published risk of bias tool to further discuss the rating system based on the information provided by the primary study authors.
Results
In December 2016, electronic and hand searches identified 1,578 articles, and of these, 56 full texts were reviewed and nine studies included. The updated searches in August 2018 retrieved 276 new articles, of which 31 full texts were read in full and one new eligible study was identified. In total, 10 studies met the inclusion criteria (Benzo et al., 2016; Bischoff et al., 2012; Cameron‐Tucker et al., 2016; Greening et al., 2014; Johnson‐Warrington, Rees, Gelder, Morgan, & Singh, 2016; Jolly et al., 2018; Khdour, Kidney, Smyth, & McElnay, 2009; Mitchell et al., 2014; Song, Yong, & Hur, 2014; Walters et al., 2013). The flow of included studies can be seen in Figure 1.
Figure 1.
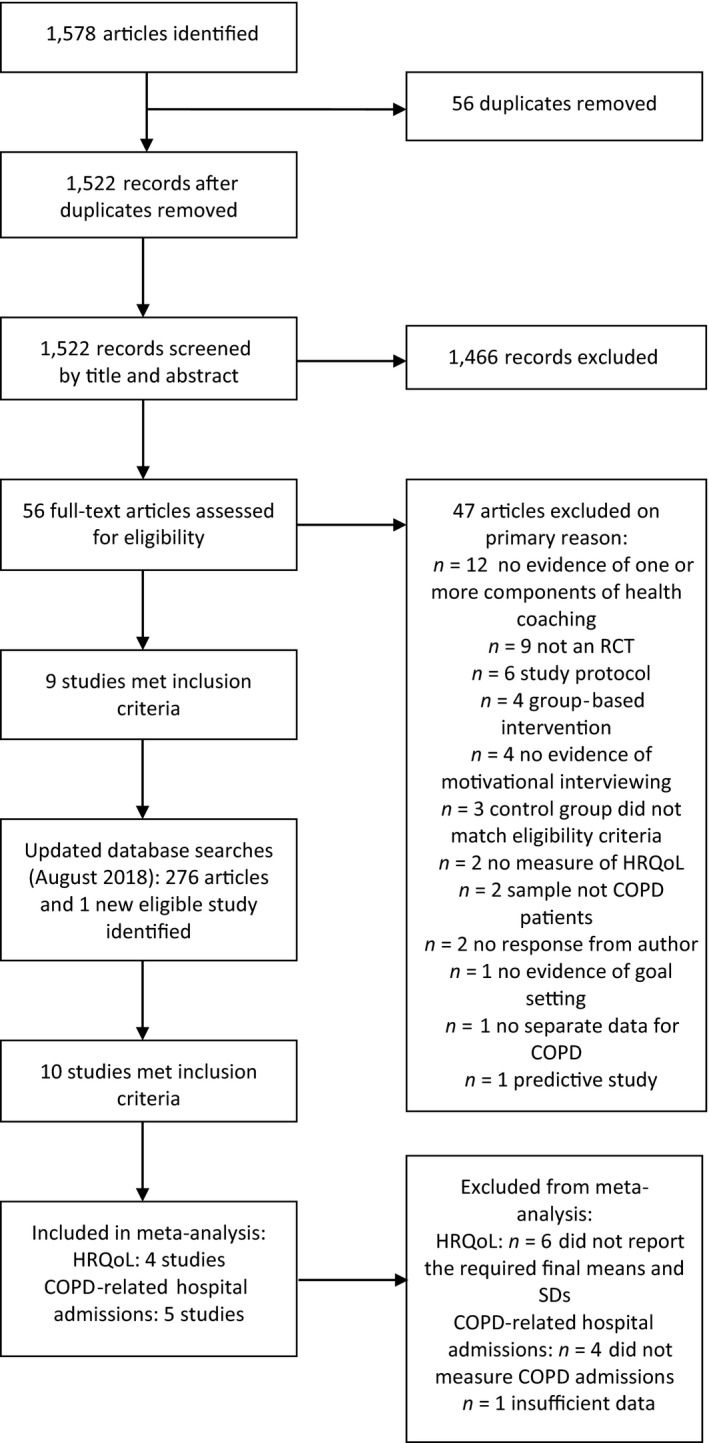
Flow diagram of study inclusion and exclusion process.
Study characteristics
The characteristics and main findings of included studies are summarized in Table 2. Table 3 presents a ‘quick look’ summary of the main findings. The 10 included studies comprised data for 1959 people with COPD, with study sample sizes ranging from 40 to 577 (mean = 196). The mean age of participants was 68 (52% male) (mean age and gender breakdown calculated from k = 9 as these details were not reported for the COPD subsample in Greening et al., 2014).
Table 2.
Main characteristics and findings of the included studies
| Authors (year) | Country | Sample: N, mean age, % male, baseline HRQoL | Intervention duration | Interventionist and delivery | Intervention details | Outcome measures and follow‐up points | Main findings |
|---|---|---|---|---|---|---|---|
| Benzo et al. (2016) | United States |
N = 215, 68 years, 45% male, moderate HRQoL |
IG: 1 × 2 hr session in person and seven telephone sessions (duration unknown) over 8 weeks CG: usual care |
The two sites had a dedicated health coach. One site also had a registered nurse and the other had a respiratory therapist | The first session was conducted in person by the health coach. The pps was given a written exacerbation emergency plan and the self‐management concepts, goal setting, action planning. The details of the forthcoming telephone sessions were discussed |
HRQoL: CRQ COPD‐related hospital admissions Physical activity: BMA 1, 3, 6, and 12 months |
HRQoL: significant between‐group differences at 6 and 12 months, favouring the IG COPD‐related hospital admissions: significantly lower at 1, 3, and 6 months in the IG, but not at 12 months Physical activity: no significant differences |
| Bischoff et al. (2012) | The Netherlands |
N = 165, 65 years, 65% male, moderate HRQoL |
IG: 2–4 × 1 hr in person sessions scheduled in 4‐6 consecutive weeks and 6 × 15 min telephone sessions over 24 months CG: (1) routine monitoring and (2) usual care |
Nurses were trained to deliver the intervention | Pps were introduced and guided through to the ‘Living well with COPD’ programme in person by the nurse. A tailored, written exacerbation plan was provided. Telephone calls were made to reinforce self‐management skills |
HRQoL: CRQ Self‐care adherence: exacerbation management 6 and 24 months |
HRQoL: no significant differences Self‐care adherence: no significant differences |
| Cameron‐Tucker et al. (2016) | Australia |
N = 65, 69 years, 45% male, moderate HRQoL |
IG: 1 × in person session (duration unknown) and a mean of 7 × 17 min telephone sessions every 7 days over 8‐12 weeks CG: usual care |
A research officer delivered the first session. Trained community nurses acted as health mentors in the telephone sessions | In the first session, goals and a personal home‐walking action plan were established, and health behaviours were discussed. Telephone calls supported action plans and other health behaviour plans |
HRQoL: CAT Physical activity: 6MWD 8‐12 weeks |
HRQoL: no significant differences Physical activity: significant between‐group difference, favouring the CG |
| Greening et al. (2014) | United Kingdom |
N = 320 with COPD (82%; from total N = 389), age, gender and baseline HRQoL scores not reported for COPD subgroup |
IG: 1 × in person session (duration unknown) and 3 × telephone sessions (duration unknown) at 48 hr, 2 weeks, and 4 weeks) CG: usual care |
The interventionist team (made up of physiotherapists and nurses) introduced pps to the SPACE for COPD manual in person and delivered the telephone sessions | MI techniques were used to introduce pps to the manual. The manual was used to structure the telephone sessions. |
HRQoL: SGRQ (at 1.5, 3, and 12 months) COPD‐related hospital admissions (at 12 months) Physical activity: ISWT and ESWT (at 1.5, 3, and 12 months) |
HRQoL: no significant differences COPD‐related hospital admissions: no significant differences Physical activity: significant between‐group difference in ESWT at 1.5 months, favouring the IG, but not at 3 or 12 months. No significant differences found in ISWT |
| Johnson‐Warrington et al. (2016) | United Kingdom |
N = 78, 68 years, 36% male, low HRQoL |
IG: 1 × 30‐45 min in person session and 6 × telephone sessions (duration unknown) over 10 weeks (within 72 hr, at 2, 4, 6, 8, and 10 weeks) CG: usual care |
A physiotherapist introduced pps to the SPACE for COPD manual. The research team delivered the telephone sessions | MI techniques were used to introduce pps to the manual and to facilitate behaviour change, goal setting, and problem‐solving. Pps worked through the manual at home. Telephone sessions were tailored to the pps needs and reinforced skills (e.g., how to identify and manage exacerbations, promote an active lifestyle, and provide encouragement) |
HRQoL: CRQ COPD‐related hospital admissions Physical activity: ISWT and ESWT Mood: HADS 3 months |
HRQoL: no significant differences. Both conditions significantly improved their CRQ score (within‐group differences) COPD‐related hospital admissions: no significant differences Physical activity: no significant differences. Both conditions significantly improved their exercise tolerance (ESWT) Mood: no significant differences |
| Jolly et al. (2018) | United Kingdom |
N = 577, 70 years, 63% male, high HRQoL |
IG: 4 × telephone sessions (21–40 min) at weeks 1, 3, 7, and 11, with supported written information at weeks 16 and 24 CG: usual care |
Nurses were trained to deliver the intervention | Telephone health coaching including self‐management skills related to health behaviours and correct inhaler use technique. A pedometer and self‐monitoring diary were provided. Standard as well as supportive, tailored written information was given after each session (e.g., goals agreed, information leaflet showing correct inhaler use technique) |
HRQoL: SGRQ, EQ‐5D‐5L (at 6 and 12 months) Physical activity: IPAQ (at 6 and 12 months), GENEActiv accelerometers (at 12 months) Mood: HADS (at 6 and 12 months) Self‐management activities related to smoking cessation, medication adherence (at 6 and 12 months) |
HRQoL: no significant differences Physical activity: significant differences in favour of the IG at 6 months, but not 12 months Physical activity: no significant differences Mood: no significant differences |
| Khdour et al. (2009) | United Kingdom |
N = 173, 66 years, 44% male, moderate HRQoL |
IG: 4 × in person sessions (the first lasted approx. 1 hr) and 2 × telephone sessions at 3 and 9 months (duration unknown) CG: usual care |
A research pharmacist delivered the first session. A clinical pharmacist then designed a tailored intervention and delivered the remaining in person and telephone sessions | Pps’ individual needs were determined in the first session. A tailored intervention was designed for the remaining sessions. Pps attended an outpatient clinic every 6 months, where self‐management education was reinforced. Pps received telephone calls between outpatient clinic appointments to reinforce COPD‐related health education, during which they were encouraged to be motivated to achieve their goals |
HRQoL: SGRQ COPD‐related hospital admissions Self‐care adherence: medication adherence, Morisky adherence scale 6 and 12 months |
HRQoL: significant differences in the symptoms domain, impact domain, and total score at 6 months, favouring the IG. Symptoms and impact domains remained significantly different at 12 months COPD‐related hospital admissions: significant difference at 6 and 12 months, indicating a reduction in hospital readmissions in the IG Self‐care adherence: the IG exhibited high adherence scores than the CG at 6 and 12 months |
| Mitchell et al. (2014) | United Kingdom |
N = 184, 69 years, 55% male, moderate HRQoL |
IG: 1 × 30–45 min in person session and 2 × telephone sessions at 2 and 4 weeks (duration unknown) CG: usual care |
A physiotherapist delivered all sessions | Pps were introduced to the SPACE for COPD manual during the initial in person session. MI techniques were used to explore the pps’ readiness to change and to enhance motivation. Pps’ needs were discussed and goal setting strategies were introduced. Pps were advised how to use the manual at home and implement the exercise regime. Skills and encouragement were given during telephone calls |
HRQoL: CRQ COPD‐related hospital admissions Physical activity: ISWT and ESWT Mood: HADS 6 weeks and 6 months |
HRQoL: significant differences in three of four CRQ domains (dyspnoea, fatigue, and emotion) at 6 weeks, favouring the IG, but not at 6 months. No significant differences for CRQ‐mastery at 6 weeks or 6 months COPD‐related hospital admissions: no significant differences Physical activity: significant differences in both the ISWT and ESWT at 6 weeks; this effect was maintained for the ESWT at 6 months Mood: significant between‐group difference in anxiety scores at 6 weeks and 6 months, favouring the IG. No significant differences in depression score |
| Song et al. (2014) | Korea |
N = 40, 67 years, 65% male, moderate HRQoL |
IG: 3 × in person sessions (duration unknown) and 2 × telephone sessions (duration unknown) over 8 weeks CG: usual care |
A nurse interventionist delivered all sessions | Each in person session consisted of self‐management education, underpinned by MI techniques, PR‐based exercises and encouragement to achieve goals |
HRQoL: SGRQ Physical activity: 6MWD Self‐care adherence: self‐report measures of medication adherence and exercise compliance 2 months |
HRQoL: significant between‐group differences in all SGRQ components, favouring the IG Physical activity: no significant differences Self‐care adherence: significant between‐group differences in medication adherence and exercise, favouring the IG. |
| Walters et al. (2013) | Australia (Tasmania) |
N = 182, 68 years, 52% male, moderate HRQoL |
IG: 16 × 30 min telephone sessions over 12 months, with increasing time between calls CG: usual care including monthly telephone calls |
A community health nurse was trained as a health mentor and delivered all sessions. | Mentors supported pps in setting medium‐ to long‐term goals targeting different health behaviours and individual action plans to reach these goals. Goals were reviewed and revised collaboratively during telephone sessions |
HRQoL: SF‐36 and SGRQ COPD‐related hospital admissions 6 months and 12 months |
HRQoL: no significant differences COPD‐related hospital admissions: no significant differences |
6MWD = 6‐min walking distance; BMA = Body Media Armband; C = control group; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; CRQ = Chronic Respiratory Questionnaire; EQ‐5D‐5L = EuroQoL 5 Dimensions 5 Levels; ESWT = endurance shuttle walk test; HADS = Hospital Anxiety and Depression Scale; HRQoL = health‐related quality of life; I = intervention group; IPAQ = International Physical Activity Questionnaire; ISWT = incremental shuttle walk test; MI = motivational interviewing; pps = participants; PR = pulmonary rehabilitation; RCT = randomized controlled trial; SF‐36; Short Form 36 Health Survey; SGRQ = St. George's Respiratory Questionnaire; SPACE = Self‐management programme of Activity = Coping and Education.
Table 3.
Summary of findings for key outcome domains
| Author (year) | HRQoL | COPD‐related hospital admissions | Physical activity | Self‐care behaviour | Mood |
|---|---|---|---|---|---|
| Benzo et al. (2016) |
CRQ 6 months: ✓ CRQ 12 months: ✓ |
1 month: ✓ 3 months: ✓ 6 months: ✓ 12 months: X |
BMA 1 month: X BMA 3 months: X BMA 6 months: X BMA 12 months: X |
n/a | n/a |
| Bischoff et al. (2012) |
CRQ 6 months: X CRQ 24 months: X |
n/a | n/a | Exacerbation management: X | n/a |
| Cameron‐Tucker et al. (2016) | CAT 2‐3 months: X | n/a | 6MWD 2‐3 months: ✓a | n/a | n/a |
| Greening et al. (2014) |
SGRQ 6 weeks: X SGRQ 3 months: X SGRQ 12 months: X |
12 months: X |
ISWT 6 weeks: X ESWT 6 weeks: ✓ ISWT 3 months: X ESWT 3 months: X ISWT 12 months: X ESWT 12 months: X |
n/a | n/a |
| Johnson‐Warrington et al. (2016) | CRQ 3 months: X | 3 months: X |
ISWT 3 months: X ESWT 3 months: X |
n/a | HADS 3 months: X |
| Jolly et al. (2018) |
EQ‐5D‐5L 6 months: X EQ‐5D‐5L 12 months: X SGRQ 6 months: X SGRQ 12 months: X |
6 months: X 12 months: X |
IPAQ 6 months: ✓ IPAQ 12 months: X GENEActiv 12 months: X |
Smoking cessation 6 months: X Smoking cessation 12 months: X Medication adherence 6 months: ✓ Medication adherence 12 months: ✓ |
HADS 6 months: X HADS 12 months: X |
| Khdour et al. (2009) |
SGRQ 6 months: ✓ SGRQ 12 months: ✓b |
6 months: ✓ 12 months: ✓ |
ESWT 3 months: X |
Medication adherence 6 months: ✓ 12 months: ✓ |
n/a |
| Mitchell et al. (2014) |
CRQ 6 weeks: ✓b
CRQ 6 months: X |
6 weeks: X 6 months: X |
ISWT 6 weeks: ✓ ESWT 6 weeks: ✓ ISWT 6 months: X ESWT 6 months: ✓ |
n/a |
HADS 6 weeks: ✓ (anxiety only) HADS 6 months: ✓ (anxiety only) |
| Song et al. (2014) | SGRQ 2 months: ✓ | n/a | 6MWD 2 months: X |
Medication adherence 2 months: ✓ Exercise compliance 2 months: ✓ |
n/a |
| Walters et al. (2013) |
SF‐36 6 months: X SGRQ 6 months: X SF‐36 12 months: X SGRQ 12 months: X |
6 months: X 12 months: X |
n/a | n/a | n/a |
6MWD, 6‐min walking distance; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; CRQ, Chronic Respiratory Questionnaire; ESWT; endurance shuttle walk test; HADS, Hospital Anxiety and Depression Scale; HRQoL, health‐related quality of life; ISWT, incremental shuttle walk test; SF‐36, Short Form 36 Health Survey; SGRQ, St. George's Respiratory Questionnaire.
In favour of the comparison group.
In some of the questionnaire domains.
The studies were published between 2009 and 2018. Five studies were conducted in the United Kingdom (Greening et al., 2014; Johnson‐Warrington et al., 2016; Jolly et al., 2018; Khdour et al., 2009; Mitchell et al., 2014), two studies in Australia (Cameron‐Tucker et al., 2016; Walters et al., 2013), one study in Korea (Song et al., 2014), one study in the Netherlands (Bischoff et al., 2012), and one study in the United States (Benzo et al., 2016). Participants were recruited in either a hospital or primary care setting. Severity of COPD at baseline was measured in nine studies, and most participants had moderate COPD.
Five studies measured respiratory‐specific HRQoL using the SGRQ (Greening et al., 2014; Jolly et al., 2018; Khdour et al., 2009; Song et al., 2014; Walters et al., 2013), four studies used the Chronic Respiratory Questionnaire (CRQ; Williams, Singh, Sewell, Guyatt, & Morgan, 2001) (Benzo et al., 2016; Bischoff et al., 2012; Johnson‐Warrington et al., 2016; Mitchell et al., 2014), and one study used the COPD Assessment Test (CAT; Jones et al., 2009) (Cameron‐Tucker et al., 2016). One study measured general HRQoL using the EuroQoL 5 Dimensions 5 Levels (EQ‐5D‐5L; Herdman et al., 2011) (Jolly et al., 2018), and one study used the 36‐Item Short Form Survey (SF‐36; Ware & Sherbourne, 1992) (Walters et al., 2013). Seven studies presented data on hospital admissions, either due to COPD or all‐cause, or both. Eight studies measured physical activity using self‐report and objective measures. Four studies measured self‐care behaviour (e.g., exacerbation management, exercise ‘compliance’, medication adherence, and smoking cessation behaviours). Three studies measured mood, each using the Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, 1983). Total follow‐up time varied between studies from 2 to 24 months (mean = 9.8 months).
At baseline, participant HRQoL was moderate for the majority of studies (Benzo et al., 2016; Bischoff et al., 2012; Cameron‐Tucker et al., 2016; Khdour et al., 2009; Mitchell et al., 2014; Song et al., 2014; Walters et al., 2013), relatively high in one study (Jolly et al., 2018), and relatively low in one study (Johnson‐Warrington et al., 2016).
Details of the intervention
Interventions were delivered by a range of health care professionals: nurse (k = 5), pharmacist (k = 1), and health coach (k = 1) (Table 2). Three studies used the Self‐Management Programme of Activity, Coping and Education (SPACE) COPD manual (Apps, Mitchell, & Harrison, 2013). Participants were introduced to the manual by a physiotherapist (k = 2) or a member of the intervention team (k = 1) and instructed to work through it independently. The average number of health coaching sessions was 7 (range = 3–16), but in two studies, the number of sessions varied between participants. In addition to health coaching, one study incorporated an inpatient pulmonary rehabilitation (PR) component with an exercise programme (Greening et al., 2014), which means it is difficult to say with confidence that any positive effects of this intervention are due to health coaching alone.
Risk of bias within studies
The results of the quality assessment are presented in Table 4. Quality varied across the studies, but risk of bias judgements was generally of low risk (69%) or unclear risk (15%). Blinding of research personnel and participants (performance bias) and blinding of outcome measure assessments (detection bias) represent the highest risk of bias domains in the included studies. In addition, three of the included studies assessed intervention fidelity. Benzo et al. (2016) and Jolly et al. (2018) achieved good fidelity to the intervention. Walters et al. (2013) reported low fidelity to the delivery of some intervention elements.
Table 4.
The risk of bias of the included studies
| Author (year) | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | ||
|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of personnel | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | ||
| Benzo et al. (2016) | + | ? | + | − | + | + | + | + |
| Bischoff et al. (2012) | + | ? | + | − | ? | + | + | + |
| Cameron‐Tucker et al. (2016) | + | + | + | − | + | + | + | + |
| Greening et al. (2014) | + | + | + | − | + | + | + | + |
| Johnson‐Warrington et al. (2016) | + | + | ? | − | + | + | + | + |
| Jolly et al. (2018) | + | + | + | − | ? | + | + | + |
| Khdour et al. (2009) | + | ? | − | − | − | + | + | + |
| Mitchell et al. (2014) | + | + | ? | − | + | + | + | + |
| Song et al. (2014) | ? | ? | ? | − | ? | + | + | + |
| Walters et al. (2013) | + | + | − | − | ? | + | + | + |
+ low risk of bias, − high risk of bias, ? unclear risk of bias.
Effect of health coaching on HRQoL
Four of 10 studies reported a significant improvement in HRQoL at follow‐up (Benzo et al., 2016; Khdour et al., 2009; Mitchell et al., 2014; Song et al., 2014). Mitchell et al. (2014) reported a significant improvement in HRQoL at 6 weeks, but not 6 months. Song et al. (2014) reported a significant improvement at 2 months follow‐up. Benzo et al. (2016) and Khdour et al. (2009) reported a significant improvement in HRQoL at 6 and 12 months after the intervention.
The remaining studies did not observe statistically significant findings. Two studies reported that the direction of effect for total HRQoL score favoured the intervention condition (Jolly et al., 2018; Johnson‐Warrington et al., 2016). Johnson‐Warrington et al. (2016) reported statistically significant within‐group differences for both conditions for all CRQ‐SR domains except CRQ emotion for the usual care condition. Between‐group differences were approaching statistical significance for CRQ dyspnoea (p = .062) and CRQ emotion (p = .077) in favour of the intervention.
Conversely, Bischoff et al. (2012) reported a reduction (−0.10) in HRQoL in the intervention condition over time, but it was not clinically significant (defined as a change in score of equal to or >0.50). Walters et al. (2013) reported that the direction of effect for total HRQoL score at 12 months favoured the usual care condition. Another study reported no within‐ or between‐group changes (Cameron‐Tucker et al., 2016), and the final study did not state the direction of effect in the COPD subsample analyses (Greening et al., 2014). Of note, Greening et al. (2014) included a PR and exercise component and it is therefore not possible to say with confidence that any positive effects of this intervention are due to health coaching alone.
Four studies were eligible to be included in the meta‐analysis for HRQoL (Jolly et al. 2018; Khdour et al., 2009; Song et al., 2014; Walters et al., 2013). Each study had used a respiratory‐specific measure of HRQoL (i.e., the SGRQ). However, Jolly et al. (2018) used the SGRQ‐C, which is a COPD‐specific version of this measure. Random effects meta‐analysis using SMD to pool effects across studies (n = 947) found a significant positive effect for health coaching on the total HRQoL, as measured by the SGRQ, when compared with treatment as usual (SMD = −0.69, 95% CI −1.28, −0.09, p = .02) (Figure 2). A considerable degree of heterogeneity was seen across the four studies (X2 = 40.30, df = 3, p = .00001; I 2 = 93%).
Figure 2.
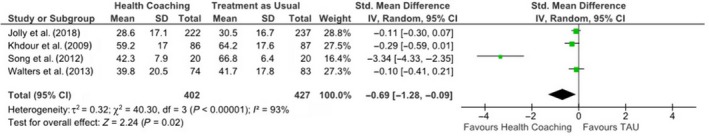
SMD and 95% CI for effect of health coaching on HRQoL in people with COPD.
Random effects meta‐analysis for the SGRQ subscales did not show any significant effects in favour of the intervention condition: for symptoms (SMD −0.50, 95% CI: −1.02, 0.03, p = .06) and activity limitation (SMD −0.13, 95% CI: −0.26, −0.01, p = .07). However, there was a significant effect in favour of health coaching on the SGRQ impact subscale (SMD −0.61, 95% CI: −1.14, −0.07, p = .03). There was considerable heterogeneity found across all subscale meta‐analyses, except for the activity subscale where heterogeneity was low (X2 = 2.67, df = 3, p = .45; I 2 = 0%).
Effect of health coaching on COPD‐related hospital admissions
Seven of 10 studies assessed the impact of health coaching on hospital admissions (Benzo et al., 2016; Greening et al., 2014; Johnson‐Warrington et al., 2016; Jolly et al. 2018; Khdour et al., 2009; Mitchell et al., 2014; Walters et al., 2013), and two of these reported significantly fewer hospital admissions in the intervention condition. Benzo et al. (2016) reported a significant reduction in COPD‐related hospital admissions in the intervention condition. This positive effect was sustained at 1, 3, and 6 months follow‐up, but not at 12 months. Khdour et al. (2009) reported a significant reduction in hospital admissions at both 6 and 12 months follow‐up.
Hospital admissions measurements were split into all‐cause hospital admissions and COPD‐related hospital admissions. Three studies (n = 470) were eligible for inclusion in random effects meta‐analysis for the effect of health coaching on all‐cause hospital admission (Khdour et al., 2009; Song et al., 2014; Walters et al., 2013). There was no significant reduction in all‐cause hospital admissions for people who received health coaching (OR = 0.70, 95% CI: 0.41–1.12, p = .20).
Five studies (n = 808) were eligible for inclusion in random effects meta‐analysis for the effect of health coaching on COPD‐related hospital admissions (Benzo et al., 2016; Johnson‐Warrington et al., 2016; Khdour et al., 2009; Mitchell et al., 2014; Walters et al., 2013). There was a significant positive effect of health coaching on COPD‐related admissions (OR = 0.45, 95% CI: 0.30, 0.67, p = .0001) (Figure 3). Heterogeneity was found to be low across these studies (X2 = 2.08, df = 4, p = .76; I 2 = 0%).
Figure 3.
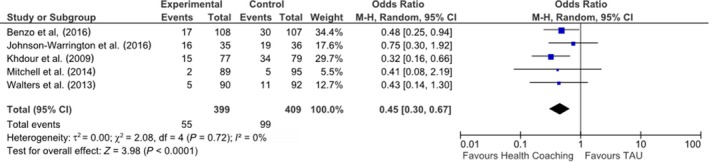
OR and 95% CI for effect of health coaching on COPD‐related hospital admissions.
Effect of health coaching on physical activity
Eight studies measured the effect of health coaching on physical activity (Benzo et al., 2016; Cameron‐Tucker et al., 2016; Greening et al., 2014; Jolly et al., 2018; Johnson‐Warrington et al., 2016; Khdour et al., 2009; Mitchell et al., 2014; Song et al., 2014). Three studies reported a significant group difference in favour of the intervention condition: at 6 weeks follow‐up (Greening et al., 2014; Mitchell et al., 2014) and at 6 months follow‐up (Jolly et al., 2018; Mitchell et al., 2014). However, one study reported a significant group difference in favour of the comparison group at 2–3 months follow‐up (Cameron‐Tucker et al., 2016). The remaining four studies reported no significant between‐group differences in physical activity at any follow‐up point.
Effect of health coaching on self‐care behaviour
Four studies measured the effect of health coaching on self‐care behaviour (Bischoff et al., 2012; Jolly et al., 2018; Khdour et al., 2009; Song et al., 2014). Three studies reported significant improvements in medication adherence in favour of the intervention condition: at 2 months follow‐up (Song et al., 2014) and at 6 and 12 months follow‐up (Jolly et al., 2018; Khdour et al., 2009). Song et al. (2014) observed a significant improvement in exercise compliance at 2 months post‐intervention. Bischoff et al. (2012) assessed exacerbation management as a self‐care behaviour, but did not observe any differences between conditions. Jolly et al. (2018) measured smoking cessation behaviours, but did not observe any differences between conditions at 6 or 12 months follow‐up.
Effect of health coaching on mood
Three studies investigated the effect of health coaching on mood in people with COPD (Jolly et al., 2018; Johnson‐Warrington et al., 2016; Mitchell et al., 2014). Mitchell et al. (2014) reported a significant improvement in the HADS anxiety subscale in favour of the intervention condition at 6 weeks follow‐up. Jolly et al. (2018) and Johnson‐Warrington et al. (2016) did not observe any significant between‐group differences at follow‐up.
Optimal mode of intervention delivery
Eight studies delivered health coaching through a combination of in person and telephone consultations. Of these eight, three studies also included the self‐led SPACE for COPD manual. In five of the eight studies, only the initial consultation was in person and the remaining sessions were telephone consultations. Two studies delivered health coaching via telephone consultations only. There was no clear pattern of results regarding whether one particular mode of delivery was more effective than another. Two of five predominantly telephone‐led health coaching interventions reported significant improvements to HRQoL, and two of four predominantly in person health coaching interventions also reported significant improvements to HRQoL. These studies all used a combination of in person and telephone sessions to deliver the intervention. The health coaching interventions delivered exclusively through telephone consultations did not report any positive effects. Therefore, the evidence for the optimal mode of delivery is inconclusive.
Discussion
This is the first review to systematically examine the effect of health coaching on HRQoL and other health outcomes in people with COPD. Our findings are not uniform; some studies found that health coaching had a positive effect on HRQoL and other studies did not. Across the studies eligible for inclusion in meta‐analysis, health coaching had a significant positive effect on HRQoL and led to a significant reduction in COPD‐related hospital admissions over 6 months. Therefore, health coaching may be a candidate intervention to improve these important outcomes in people with COPD.
We will now discuss these findings in the light of the limitations of the available evidence. Our findings are over a relatively short‐term follow‐up period. Only one included study measured outcomes after 12 months and reported no effect of health coaching on patient outcomes. Thus, the available evidence for longer‐term improvements in COPD health outcomes is limited. The evidence for improvements to outcomes such as physical activity and mood is inconsistent.
Our meta‐analysis included two of the four studies that reported a positive effect of health coaching on HRQoL and showed a pooled positive effect of health coaching on total HRQoL as well as the SGRQ impact subscale (Khdour et al., 2009; Song et al., 2014). It is important to note that, while a significant overall positive effect was found using the SGRQ scale, not all of the subscales demonstrated significant differences. It may be the case that, following health coaching, positive changes are more likely in some subscales than others (e.g., the impact subscale, measuring psychosocial disruption, rather than the symptoms subscale, measuring the severity of respiratory symptoms) and that these differences are sufficient to demonstrate an overall effect.
Our findings echo those of published systematic reviews of health coaching for people with other LTCs (Kivelä et al., 2014) and for supporting various health behaviours that affect patient health outcomes (Olsen & Nesbitt, 2010). There are a number of possible reasons why the remaining six studies did not observe positive effects of health coaching. For example, the number and frequency of coaching sessions may affect the extent to which people with COPD engage with the intervention content and feel supported. Further, the goals set to overcome barriers may be so difficult for people with COPD to achieve that a relatively short‐term health coaching intervention may not be sufficient to establish any real and lasting change. One of the six studies reported relatively low HRQoL in participants at baseline. It may be the case that improving HRQoL in participants with already low‐scoring HRQoL presents greater or additional challenges compared with participants who begin the intervention with a higher baseline HRQoL score.
Across the studies reporting a positive effect of health coaching on HRQoL and included in our meta‐analysis, the between‐group difference on the SGRQ exceeded the minimum clinically important difference (MCID) for that particular scale (Jones, 2005; Jones et al., 2014). However, this was not the case for the two studies that did not find a statistically significant effect, as the difference between the health coaching and treatment as usual group did not meet the level of the MCID. This was also the case across studies using alternative HRQoL measures. For example, Bischoff et al. (2012) reported a reduction on the CRQ, but this did not reach the level MCID for that scale (Schünemann, Puhan, Goldstein, Jaeschke, & Guyatt, 2005). Therefore, it is unclear whether participants who received health coaching would have noticed a clinically significant improvement in their day‐to‐day lives, compared with those participants in the treatment as usual group. We did not look at within‐group changes from baseline to follow‐up, which would have allowed further interpretation of the MCID.
Few studies included in this review specifically measured mood. Depression and anxiety are highly prevalent in people with COPD, and depression has been shown to predict poor HRQoL (Blakemore et al., 2014) and to negatively impact on self‐care and treatment adherence (DiMatteo, Lepper, & Croghan, 2000). Depression also predicts the use of urgent care in people with LTCs (Dickens et al., 2012; Guthrie et al., 2016). Support for depression and anxiety may therefore need to be a more significant part of health coaching interventions for people with COPD in future. The success of such support for low mood will need to be tested in models of health coaching to account for the considerable impact of depression on COPD outcomes. A previous systematic review of health coaching in LTCs found the intervention positively impacted psychological outcomes such as self‐efficacy and mental health status (Kivelä et al., 2014), suggesting there may be a promising role for health coaching in improving mental health outcomes for people with COPD. Investigating the impact of health coaching on mood (e.g., depression and anxiety) may therefore be a worthwhile avenue for future research.
Based on our meta‐analysis results, we found that health coaching significantly reduces COPD‐related hospital admissions. It may be the case that the increased awareness of and attention to self‐management strategies that health coaching instils are important for motivating people to take up behaviours that translate into improvement of outcomes such as hospital admissions. Currently, it is estimated that COPD costs over £800 million per year in the United Kingdom and much of this cost is accounted for by attendance at accident and emergency and emergency admissions (British Lung Foundation, 2016; Department of Health, 2012). Therefore, any intervention that has the potential to improve self‐management, impact HRQoL, and reduce urgent health care use is of interest to health care providers and health economists alike.
There are several strengths of this review. Firstly, it is the first systematic review of published RCTs assessing the impact of health coaching for people with COPD and thus furthers current understanding of the utility of health coaching. Health coaching has been generally poorly defined (Wolever et al., 2013), and this review has applied a cogent definition rigorously. This somewhat conservative approach ensures findings reviewed are from core health coaching studies. We followed established, explicit, and reproducible procedures for conducting a systematic review. The inter‐rater reliability of the study inclusion process was of high agreement, thus minimizing the chance of selection bias, and the quality assessment was second‐coded, which increases methodological rigour (Centre for Reviews & Dissemination, 2009). We followed the PRISMA guidelines for reporting systematic reviews. The included studies were peer‐reviewed RCTs and of reasonable quality.
This review has some limitations. We have reviewed a small number of studies, and we have not assessed publication bias. We were only able to include a minority of the eligible studies in the meta‐analysis for HRQoL and hospital admissions and, therefore, these results must be interpreted with appropriate caution. As per Cochrane guidelines, five studies were excluded from the meta‐analysis of the effects of health coaching on HRQoL on the basis that they presented change scores as opposed to final means and standard deviations (Higgins & Green, 2011). One further study was excluded as there were insufficient data presented.
Across most domains, the studies included in the meta‐analysis were generally of a low risk of bias. However, the studies were at some risk of bias due to lack of blinding of participants. This is somewhat difficult to avoid due to the nature of the intervention. Furthermore, the study conducted by Song et al. (2014) and included in the meta‐analysis is shown in the forest plot as a clear outlier, demonstrating a very positive effect of health coaching on HRQoL. One possible explanation for this is that the follow‐up period used by Song et al. was shorter than that used in the other studies (2 months rather than 6 months). It is therefore possible that more positive effects are seen closer to the end of treatment when the intervention is fresh to the participants and that these effects deteriorate over time, as symptoms also increase in severity of this progressively worsening condition. In order to explore this, we reran the meta‐analysis excluding the study by Song et al. (2014). We found that the effect on HRQoL was smaller (SMD −0.15), but still significant. This demonstrates that the significant effect of health coaching on HRQoL in COPD is not solely due to this outlier study. As per our protocol, we kept Song et al. in our meta‐analysis.
The present review is limited by the relatively short follow‐up points employed in the primary studies; no conclusions for longer‐term effects can be drawn. Further, data to be included in the meta‐analysis were only available across studies at 6 months. A core ethos of health coaching is to be patient‐centred and tailored. This increases heterogeneity in intervention components and outcome assessments, as well as the challenge of synthesizing evidence in meta‐analysis. Other systematic reviews report a similar heterogeneity (Dejonghe, Becker, Froboese, & Schaller, 2017; Hill, Richardson, & Skouteris, 2015; Wolever et al., 2013). While it was possible to establish whether studies included the key components of health coaching, intervention content was generally poorly reported.
This review adds to the current literature by presenting an overview of the most up‐to‐date research of health coaching for people with COPD on a range of health outcomes. Our findings are of relevance to researchers interested in self‐management interventions for people with COPD, HRQoL, hospital admissions, cost‐effective health care, physical activity, self‐care behaviours, and mood. Future health coaching interventions aimed at supporting these health outcomes in this population (or others) will benefit from better exploring the mechanisms through which health coaching has an effect. It is not possible to tell from this review what unique contribution each component of health coaching made to the intervention's success, or whether one ‘active ingredient’ of health coaching is more effective than other components at bringing about positive change in health outcomes. Given the diversity in health coaching interventions (Wolever et al., 2013), an evidence‐based assessment of the most effective health coaching interventions for people with COPD may be a worthwhile route for research. Specifically, examining the moderating impact of the presence or absence of intervention components and intervention features upon effect sizes may usefully indicate the critical components of an effective health coaching intervention. This will only be possible if intervention content is reported in sufficient detail to allow researchers to appreciate subtle yet key differences in content. Further, as only a minority of studies assessed intervention fidelity, it will be important to ensure that health coaching interventions are delivered as intended and that this is assessed in both the intervention and control group arms (e.g., to ensure no ‘leakage’ of intervention content to control participants), in order to establish that any observed effects are truly due to health coaching.
The interventions included in the present review were delivered by a range of health care professionals (the majority were nurses) and via a combination of in person and telephone consultations, which makes it difficult to draw any robust conclusions regarding the best delivery modality. Previous studies of health coaching for people with LTCs have shown that the greatest effects were observed for interventions in which the coaches were trained psychologists (Sacco, Malone, Morrison, Friedman, & Wells, 2009; Wolever et al., 2010), health lifestyle coaches (Hersey et al., 2012) or educated coaches (Linden, Butterworth, & Prochasca, 2010). Further, Thom et al. (2016) developed a conceptual model of how health coaching can support people in making health‐related decisions and behaviour change. The key focus of this model is for health coaches to establish a trusting relationship with the individual, which is underpinned by education, personal support, practical support, and the ability of the health coach to act as a bridge between patients and clinicians. In order to implement health coaching within any health care system, it remains important to identify who is best placed to deliver the intervention and how it should be delivered.
Conclusion
Health coaching may have the potential to improve HRQoL and reduce COPD‐related hospital admissions for people with COPD. While this review suggests health coaching is a candidate intervention, the high heterogeneity of studies makes determining the exact effect of health coaching difficult. Future research should aim to identify how and by whom health coaching is most effective when delivered and establish the extent to which improved HRQoL may lead to other important health improvements in COPD, such as depression.
Funding sources
The British Psychological Society provided funds for publishing the Open Access version of this article.
Conflict of interest
All authors declare no conflict of interest.
Supporting information
Appendix S1. PRISMA 2009 checklist.
Appendix S2. Search strategy.
Acknowledgements
HL is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC) (funding reference number: IS‐BRC‐1215‐20007). We would like to acknowledge the authors of the primary studies who responded to our queries about study eligibility.
References
- Almagro, P. , Barreiro, B. , de Echagüen, A. O. , Quintana, S. , Carballeira, M. R. , Heredia, J. L. , & Garau, J. (2006). Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration, 73, 311–317. 10.1159/000088092 [DOI] [PubMed] [Google Scholar]
- Apps, L. , Mitchell, K. , & Harrison, S. (2013). The development and pilot testing of the self‐management program of activity, coping and education for chronic obstructive pulmonary disease (SPACE for COPD). International Journal of Chronic Obstructive Pulmonary Disease, 8, 317–327. 10.2147/COPD.S40414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas, T. , McLennon, S. M. , Carpenter, J. S. , Buelow, J. M. , Otte, J. L. , Hanna, K. M. , … Welch, J. L. (2012). Systematic review of health‐related quality of life models. Health and Quality of Life Outcomes, 10(1), 134 10.1186/1477-7525-10-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow, J. , Wright, C. , Sheasby, J. , Turner, A. , & Hainsworth, J. (2002). Self‐management approaches for people with chronic conditions: A review. Patient Education and Counselling, 48, 177–187. 10.1177/1359105305057320 [DOI] [PubMed] [Google Scholar]
- Benzo, R. , Vickers, K. , Novotny, P. J. , Tucker, S. , Hoult, J. , Neuenfeldt, P. , … McEvoy, C. (2016). Health coaching and chronic obstructive pulmonary disease rehospitalization. A randomized study. American Journal of Respiratory and Critical Care Medicine, 194, 672–680. 10.1164/rccm.201512-2503OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, E. W. , Akkermans, R. , Bourbeau, J. , van Weel, C. , Vercoulen, J. H. , & Schermer, T. R. (2012). Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: Randomised controlled trial. British Medical Journal, 345, e7642 10.1136/bmj.e7642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore, A. , Dickens, C. , Guthrie, E. , Bower, P. , Kontopantelis, E. , Afzal, C. , & Coventry, P. A. (2014). Depression and anxiety predict health‐related quality of life in chronic obstructive pulmonary disease: Systematic review and meta‐analysis. International Journal of Chronic Obstructive Pulmonary Disease, 9, 501–512. 10.2147/COPD.S58136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein, M. , Hedges, L. , Higgins, J. , & Rothstein, H. (2009). Introduction to meta‐analysis (1st ed.). New York, NY: Wiley. [Google Scholar]
- British Lung Foundation (2016). Chronic obstructive pulmonary disease (COPD) statistics. Retrieved from https://statistics.blf.org.uk/copd
- Cameron‐Tucker, H. L. , Wood‐Baker, R. , Joseph, L. , Walters, J. A. , Schüz, N. , & Walters, E. H. (2016). A randomized controlled trial of telephone‐mentoring with home‐based walking preceding rehabilitation in COPD. International Journal of Chronic Obstructive Pulmonary Disease, 11, 1991–2000. 10.2147/COPD.S109820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, D. , Buys, N. , Sriram, K. B. , Sharma, S. , Morris, N. , & Sun, J. (2016). The effects of chronic obstructive pulmonary disease self‐management interventions on improvement of quality of life in COPD patients: A meta‐analysis. Respiratory Medicine, 121, 81–90. 10.1016/j.rmed.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination (2009). Systematic reviews: CRD's guidance for undertaking reviews in health care. York, UK: University of York: Centre for Reviews & Dissemination. [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioural sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- da Silva, D. (2011). Helping people help themselves: A review of the evidence considering whether it is worthwhile to support self‐management. London, UK: Health Foundation; Retrieved from https://www.health.org.uk/sites/health/files/HelpingPeopleHelpThemselves.pdf [Google Scholar]
- Dejonghe, L. A. L. , Becker, J. , Froboese, I. , & Schaller, A. (2017). Long‐term effectiveness of health coaching in rehabilitation and prevention: A systematic review. Patient Education and Counselling, 100, 1643–1653. 10.1016/j.pec.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Department of Health (2012). An outcomes strategy for COPD and asthma: NHS companion document. Retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216531/dh_134001.pdf
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7, 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Dickens, C. , Katon, W. , Blakemore, A. , Khara, A. , McGowan, L. , Tomenson, B. , … Guthrie, E. (2012). Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta‐analysis. Journal of Psychosomatic Research, 73, 334–342. 10.1016/j.jpsychores.2012.08.018 [DOI] [PubMed] [Google Scholar]
- DiMatteo, M. R. , Lepper, H. S. , & Croghan, T. W. (2000). Depression is a risk factor for noncompliance with medical treatment: Meta‐analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine, 160, 2101–2107. 10.1001/archinte.160.14.2101 [DOI] [PubMed] [Google Scholar]
- Domingo‐Salvany, A. , Lamarca, R. , Ferrer, M. , Garcia‐Aymerich, J. , Alonso, J. , Félez, M. , … Antó, J. (2002). Health‐related quality of life and mortality in male patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 166, 680–685. 10.1164/rccm.2112043 [DOI] [PubMed] [Google Scholar]
- Garrido, P. , Díez, J. , Gutiérrez, J. , Centeno, A. , Vázquez, E. , Miguel, Á. , … García, R. (2006). Negative impact of chronic obstructive pulmonary disease on the health‐related quality of life of patients. Results of the EPIDEPOC study. Health and Quality of Life Outcomes, 4, 31 10.1186/1477-7525-4-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2017). Global strategy for the diagnosis, management and prevention of COPD. Retrieved from http://goldcopd.org
- Greening, N. J. , Williams, J. E. , Hussain, S. F. , Harvey‐Dunstan, T. C. , Bankart, M. J. , Chaplin, E. J. , … Steiner, M. C. (2014). An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: Randomised controlled trial. British Medical Journal, 349, g4315 10.1136/bmj.g4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, E. A. , Dickens, C. , Blakemore, A. , Watson, J. , Chew‐Graham, C. , Lovell, K. , … Tomenson, B. (2016). Depression predicts future emergency hospital admissions in primary care patients with chronic physical illness. Journal of Psychosomatic Research, 82, 54–61. 10.1016/j.jpsychores.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman, M. , Gudex, C. , Lloyd, A. , Janssen, M. F. , Kind, P. , Parkin, D. , … Badia, X. (2011). Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Quality of Life Research, 20, 1727–1736. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey, J. , Khavjou, O. , Strange, L. , Atkinson, R. , Blair, S. , Campbell, S. , … Britt, M. (2012). The efficacy and cost‐effectiveness of a community weight management intervention: A randomised controlled trial of the health weight management demonstration. Preventative Medicine, 54, 42–49. 10.1016/j.ypmed.2011.09.018 [DOI] [PubMed] [Google Scholar]
- Higgins, J. , Altman, D. , Gøtzsche, P. , Jüni, P. , Moher, D. , Oxman, A. , … Sterne, J. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. British Medical Journal, 343, d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. , & Green, S. (2011). Cochrane handbook for systematic reviews of interventions [Version 5.1.0]. Retrieved from www.cochrane-handbook.org
- Hill, B. , Richardson, B. , & Skouteris, H. (2015). Do we know how to design effective health coaching interventions: A systematic review of the state of the literature. American Journal of Health Promotion, 29, e158–e168. 10.4278/AJHP.130510-LIT-238 [DOI] [PubMed] [Google Scholar]
- Hu, J. , & Meek, P. (2005). Health‐related quality of life in individuals with chronic obstructive pulmonary disease. Heart & Lung: The Journal of Acute and Critical Care, 34, 415–422. 10.1016/j.hrtlng.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Johnson‐Warrington, V. , Rees, K. , Gelder, C. , Morgan, M. D. , & Singh, S. J. (2016). Can a supported self‐management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease, 11, 1161–1169. 10.2147/COPD.S91253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly, K. , Sidhu, M. S. , Hewitt, C. A. , Coventry, P. A. , Daley, A. , Jordan, R. , Fitzmaurice, D . (2018). Self management of patients with mild COPD in primary care: Randomised controlled trial. British Medical Journal, 361, k2241 10.1136/bmj.k2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, P. (1991). Quality of life measurement for patients with diseases of the airways. Thorax, 46, 676–682. 10.1136/thx.46.9.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, P. W. (2005). St. George's respiratory questionnaire: MCID. COPD: Journal of Chronic Obstructive Pulmonary Disease, 2(1), 75–79. 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- Jones, P. W. , Beeh, K. M. , Chapman, K. R. , Decramer, M. , Mahler, D. A. , & Wedzicha, J. A. (2014). Minimal clinically important differences in pharmacological trials. American Journal of Respiratory and Critical Care Medicine, 189, 250–255. 10.1136/thx.46.9.67610.1164/rccm.201310-1863PP [DOI] [PubMed] [Google Scholar]
- Jones, P. , Brusselle, G. , Dal Negro, R. , Ferrer, M. , Kardos, P. , Levy, M. , … Banik, N. (2011). Properties of the COPD assessment test in a cross‐sectional European study. European Respiratory Journal, 38(1), 29–35. 10.1183/09031936.00177210 [DOI] [PubMed] [Google Scholar]
- Jones, P. W. , Harding, G. , Berry, P. , Wiklund, I. , Chen, W. H. , & Leidy, N. K. (2009). Development and first validation of the COPD Assessment Test. European Respiratory Journal, 34, 648–654. 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- Jones, P. W. , Quirk, F. H. , & Baveystock, C. M. (1991). The St George's respiratory questionnaire. Respiratory Medicine, 85, 25–31. 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- Khdour, M. R. , Kidney, J. C. , Smyth, B. M. , & McElnay, J. C. (2009). Clinical pharmacy‐led disease and medicine management programme for patients with COPD. British Journal of Clinical Pharmacology, 68, 588–598. 10.1111/J.1365-2125.2009.03493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä, K. , Elo, S. , Kyngäs, H. , & Kääriäinen, M. (2014). The effects of health coaching on adult patients with chronic diseases: A systematic review. Patient Education and Counselling, 97, 147–157. 10.1016/j.pec.2014.07.026 [DOI] [PubMed] [Google Scholar]
- Lau, J. , Ioannidis, J. P. , Terrin, N. , Schmid, C. H. , & Olkin, I. (2006). Evidence based medicine: The case of the misleading funnel plot. British Medical Journal, 333, 597–600. 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden, A. , Butterworth, S. , & Prochasca, J. (2010). Motivational interviewing‐based health coaching as a chronic care intervention. Journal of Evaluation in Clinical Practice, 16, 166–174. 10.1111/j.1365-2753-2009.01300.x [DOI] [PubMed] [Google Scholar]
- Linder, H. , Menzies, D. , Kelly, J. , Taylor, S. , & Shearer, M. (2003). Coaching for behaviour change in chronic disease: A review of the literature and the implications for coaching as a self‐management intervention. Australian Journal of Primary Health, 9, 177–185. 10.1071/PY03044 [DOI] [Google Scholar]
- Mitchell, K. E. , Johnson‐Warrington, V. , Apps, L. D. , Bankart, J. , Sewell, L. , Williams, J. E. , … Singh, S. J. (2014). A self‐management programme for COPD: A randomised controlled trial. European Respiratory Journal, 44, 1538–1547. 10.1183/09031936.00047814 [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & Prisma Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6, e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monninkhof, E. , van der Valk, P. D. L. P. M. , Van der Palen, J. , Van Herwaarden, C. , Partridge, M. R. , & Zielhuis, G. (2003). Self‐management education for patients with chronic obstructive pulmonary disease: A systematic review. Thorax, 58, 394–398. 10.1136/thorax.58.5.394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, J. M. , & Nesbitt, B. J. (2010). Health coaching to improve healthy lifestyle behaviors: An integrative review. American Journal of Health Promotion, 25(1), e1–e12. 10.4278/ajhp.090313-LIT-101 [DOI] [PubMed] [Google Scholar]
- Osman, I. , Godden, D. , Friend, J. , Legge, J. , & Douglas, J. (1997). Quality of life and hospital re‐admission in patients with chronic obstructive pulmonary disease. Thorax, 52(1), 67–71. 10.1136/thx.52.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, S. , Blakemore, A. , & Long, H. (2016). A systematic review of the effectiveness of health coaching as an intervention for patients with chronic obstructive pulmonary disease. PROSPERO 2016 CRD42016050329. Retrieved from http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016050329
- Peytremann‐Bridevaux, I. , Staeger, P. , Bridevaux, P. , Ghali, W. , & Burnand, B. (2008). Effectiveness of chronic obstructive pulmonary disease‐management programs: Systematic review and meta‐analysis. The American Journal of Medicine, 121, 433–443. 10.1016/j.amjmed.2008.02.009 [DOI] [PubMed] [Google Scholar]
- Roberts, C. M. , Lowe, D. , Bucknall, C. E. , Ryland, I. , Kelly, Y. , & Pearson, M. G. (2002). Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax, 57, 137–141. 10.1136/thorax.57.2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco, W. , Malone, J. , Morrison, A. , Friedman, A. , & Wells, K. (2009). Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. Journal of Behavioural Medicine, 32, 349–359. 10.1007/s10865-009-9209-4 [DOI] [PubMed] [Google Scholar]
- Schünemann, H. J. , Puhan, M. , Goldstein, R. , Jaeschke, R. , & Guyatt, G. H. (2005). Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ). COPD: Journal of Chronic Obstructive Pulmonary Disease, 2(1), 81–89. 10.1081/COPD-200050651 [DOI] [PubMed] [Google Scholar]
- Seemungal, T. A. , Donaldson, G. C. , Paul, E. A. , Bestall, J. C. , Jeffries, D. J. , & Wedzicha, J. A. (1998). Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 157, 1418–1422. 10.1164/ajrccm.157.5.9709032 [DOI] [PubMed] [Google Scholar]
- Song, H. Y. , Yong, S. J. , & Hur, H. K. (2014). Effectiveness of a brief self‐care support intervention for pulmonary rehabilitation among the elderly patients with chronic obstructive pulmonary disease in Korea. Rehabilitation Nursing, 39, 147–156. 10.1002/rnj.92 [DOI] [PubMed] [Google Scholar]
- The King's Fund (2012). From vision to action: Making patient‐centred care a reality. Retrieved from www.kingsfund.org.uk/sites/default/files/field/field_publication_file/Richmond-group-from-vision-to-action-April-2012-1.pdf
- Thom, D. H. , Wolf, J. , Gardner, H. , DeVore, D. , Lin, M. , Ma, A. , … Saba, G. (2016). A qualitative study of how health coaches support patients in making health‐related decisions and behavioural changes. The Annals of Family Medicine, 14, 509–516. 10.1370/afm.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, T. , Barber, R. M. , Bell, B. , Bertozzi‐Villa, A. , Biryukov, S. , Bolliger, I. , … Duan, L. (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 386, 743–800. 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, J. A. , Cameron‐Tucker, H. , Courtney‐Pratt, H. , Nelson, M. , Robinson, A. , Scott, J. , … Wood‐Baker, R. (2012). Supporting health behaviour change in chronic obstructive pulmonary disease with telephone health‐mentoring: Insights from a qualitative study. BMC Family Practice, 13(1), 55 10.1186/1471-2296-13-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, J. , Cameron‐Tucker, H. , Wills, K. , Schüz, N. , Scott, J. , Robinson, A. , … Walters, E. H. (2013). Effects of telephone health mentoring in community‐recruited chronic obstructive pulmonary disease on self‐management capacity, quality of life and psychological morbidity: A randomised controlled trial. British Medical Journal Open, 3, e003097 10.1136/bmjopen-2013-003097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, J. E. , & Sherbourne, C. D. (1992). The MOS 36‐item Short‐Form Health Survey (SF‐36): I. Conceptual framework and item selection. Medical Care, 30, 473–483. 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- Williams, J. E. , Singh, S. J. , Sewell, L. , Guyatt, G. H. , & Morgan, M. D. (2001). Development of a self‐reported Chronic Respiratory Questionnaire (CRQ‐SR). Thorax, 56, 954–959. 10.1136/thorax.56.12.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolever, R. , Dreusicke, M. , Fikkan, J. , Hawkins, T. , Yeung, S. , Wakefield, J. , … Skinner, E. (2010). Integrative health coaching for patients with type 2 diabetes: A randomized clinical trial. Diabetes Education, 36, 629–639. 10.1177/0145721710371523 [DOI] [PubMed] [Google Scholar]
- Wolever, R. , Simmons, L. , Sforzo, G. , Dill, D. , Kaye, M. , Bechard, E. , … Yang, N. (2013). A systematic review of the literature on health and wellness coaching: Defining a key behavioral intervention in healthcare. Global Advances in Health and Medicine, 2(4), 38–57. 10.7453/gahmj.2013.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (2016). Chronic respiratory diseases. Burden of COPD. Retrieved from http://www.who.int/respiratory/copd/burden/en/
- Zigmond, A. S. , & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67, 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. PRISMA 2009 checklist.
Appendix S2. Search strategy.


