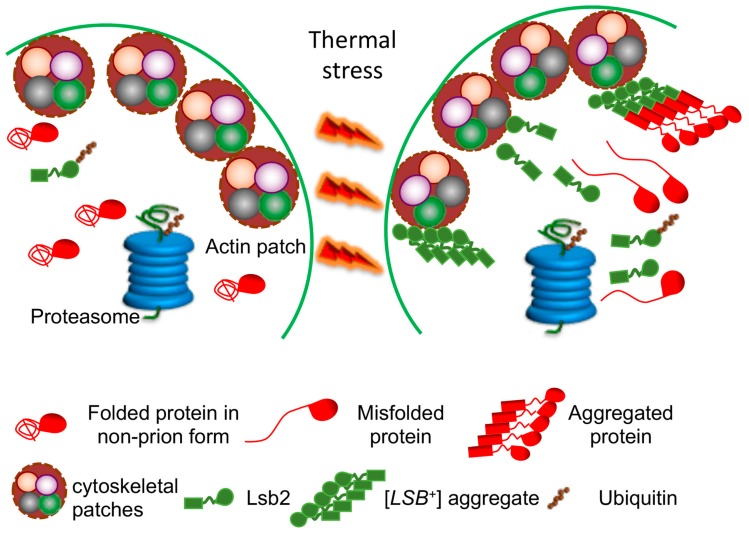
Figure 2.
Lsb2 aggregation and prion formation during thermal stress. Thermal stress (39 °C) leads to an increased synthesis of the Lsb2 protein, as well as to misfolding of other proteins. When present at high concentration, Lsb2 forms prion-like aggregates ([LSB+]), which are associated with peripheral cytoskeletal patches and promote assembly of misfolded proteins into protective (but potentially amyloidogenic) aggregate deposits. [LSB+] aggregates are metastable and lost in cell divisions after stress, while the Lsb2 protein is ubiquitinated and degraded by a proteasome.