Figure 1.
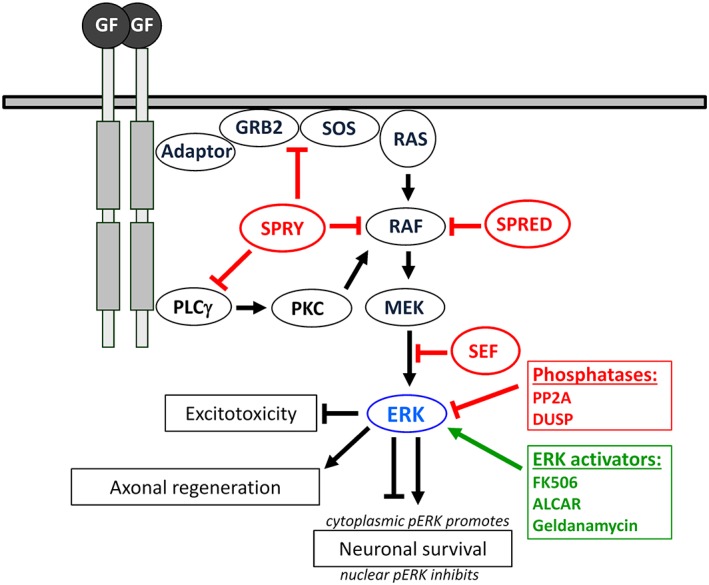
The RAS/RAF/MEK/ERK pathway: Upon growth factor (GF) activation RTKs auto‐phosphorylate and recruit the SH2 domain‐containing GRB2, which interacts with the guanine nucleotide exchange factor SOS that induces release of GDP from RAS, which subsequently binds GTP. RAS recruits RAF to the membrane. Active RAF then acts on MEK dual specificity kinase that phosphorylates ERK on both threonine and tyrosine residues. The dimerized form of ERK exerts a variety of post‐transcriptional but also nuclear effects by phosphorylation of transcription factors. The RAS/ERK pathway is tightly regulated. Several phosphatases including PP2A and DUSPs inactivate ERK in the cytoplasm or nucleus. Furthermore, endogenous modulators such as SPRY, SPRED, or SEF inhibit ERK signaling upstream and downstream of RAS. Activating drugs such as FK506, ALCAR, or Geldanamycin enhance ERK signaling. Increased pERK levels promote peripheral axon regeneration and neuronal survival but inhibit excitotoxicity. Downregulation of ERK inhibitors such as SPRY2 or treatment with ERK activators like FK506 has been demonstrated to promote peripheral nerve regeneration as well.
