Figure 2.
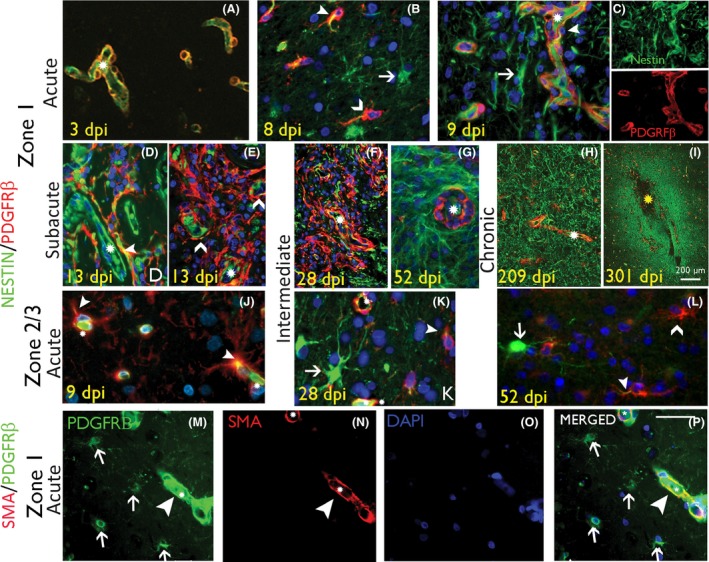
Pericytes and nestin‐expressing reactive glial cell types in intracranial recording (ICR) injuries of different stages. (A–C) Nestin/PDGFRβ in acute injuries in Zone 1. (A) At 3 days post injury (dpi) nestin labelling was relatively confined to vessel endothelium and PDGFRβ in pericytes, forming an incomplete layer around the endothelium. (B‐C) At 8 to 9 dpi, increased nestin expression in perivascular, reactive and bipolar stromal cells (arrows) with increased PDGFRβ cells around small vessels (chevrons), some with focal nestin co‐expression (arrowhead; asterisks indicate capillary lumen); In (C) the nestin and PDGFRβ are also shown as single channels. (D, E) Nestin/PDGFRβ in subacute injuries in Zone 1. (D) At this stage nestin expression is noted in elongated processes and reactive, bipolar and multipolar cells between capillaries and co‐expression with PDGFRβ is observed (arrowhead; split channel images shown in Figure S2). (E) ‘Lace‐like’ proliferations of PDGFRβ+ cells lifting away from the vasculature (asterisk) is noted (chevrons) (split channels shown in Figure S2). (F) Nestin/PDGFRβ in intermediate‐age injuries in Zone 1. By this stage more prominent increase in the number and networks of PDGFRβ+ cells in Zone 1 is noted, not associated with vessels (asterisk) and with some focal nestin co‐expression (split channels shown in Figure S2); these cells declined in number with age and, in (G) shown at 52 dpi, PDGFRβ expression is more limited to vasculature (asterisk). (H and I) Nestin/PDGFRβ in chronic injuries in Zone 1 at 209 and 301 dpi respectively, shows a residual increase in nestin+ fibrous processes, demarcating the scar site whereas PDGFRβ expression is mainly perivascular. (J) Nestin/PDGFRβ in acute injuries in Zone 3 in acute (8 dpi) and intermediate phases (K 28 dpi and L 52 dpi) shows multipolar cells that are nestin+ (arrow), PDGFRβ+ (chevron) or show double labelling (arrowhead) (asterisks indicate capillary) (J–L : shown at higher magnification in Figure S2C’). (M–P) SMA/PDGFRβ double labelling in Zone 1 confirms co‐localization in pericytic cells surrounding capillaries (arrowheads) but not in small multipolar PDGBRβ+ cells (arrows). In all figures, asterisks denote capillary vascular channels, arrowheads denote double‐labelled cells and arrows and chevrons denote single labelled cells as indicated. Scale bar shown in P is equivalent to 500 microns in A–G, 1000 and 20 microns in J to P.
