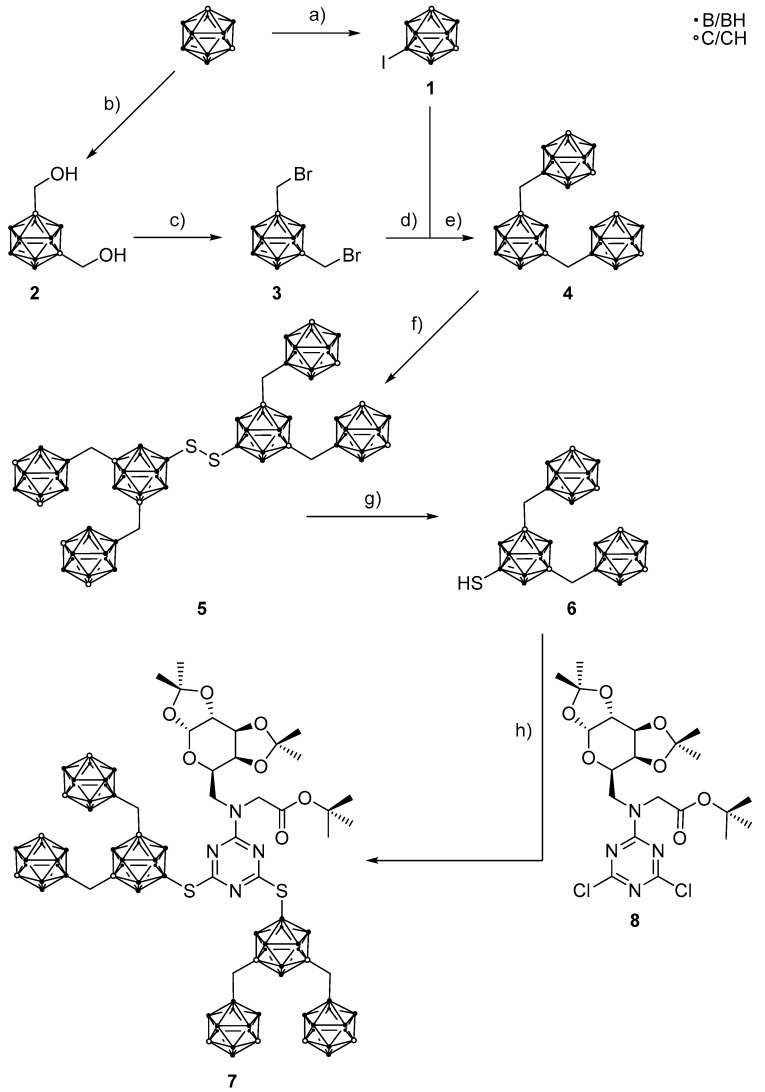
Scheme 1.
Preparation of tert-butyl-N-{4,6-bis[(1,7-dicarba-closo-dodecaboran-9-ylmethyl)1,7-dicarba-closo-dodecaboran-9-ylthio]-1,3,5-triazin-2-yl}-N-(1′,2′:3′,4′-di-O-isopropylidene-6′-deoxy-α-d-galacto-pyranos-6′-yl)glycinate (7). a) I2, AlCl3, CH2Cl2, rt, 2 d, 92% [62,63]; b) paraformaldehyde, n-BuLi, tetrahydrofuran (THF), rt, overnight, 89%; c) Br2, PPh3, benzene, reflux, 46 h, 95% [64,65,66]; d) from 3—Mg, THF, reflux, 2 h; e) from 1—11 mol% CuI, 4 mol% [PdCl2(PPh3)2], THF, reflux, 2 d, 30% [61,62,63,64,67,68]; f) S2Cl2, AlCl3, CH2Cl2, reflux, 4 h, 67% [69]; g) Zn, HClconc., glacial acetic acid (HOAc), ethyl acetate, reflux, 4 h, 81% [69]; h) +8, K2CO3, MeCN, reflux, 2 d, 79%.