Table 1.
Optimization of reaction conditionsa
 | |||
|---|---|---|---|
| Entry | Base | Temp. (°C) | Yieldb (%) |
| 1c,d | 2-Chloropyridine | −10 | 36 |
| 2d | 2-Chloropyridine | −10 | 56 |
| 3 | 2-Chloropyridine | −10 | 61 |
| 4 | 2-Fluoropyridine | −10 | 45 |
| 5 | Pentafluoropyridine | −10 | 38 |
| 6 | 2-Methoxypyridine | −10 | 0 |
| 7 | Pyridine | −10 | 21 |
| 8 | None | −10 | 34 |
| 9 | 2-Chloropyridine | −41 | 84 |
| 10 | 2-Chloropyridine | −78 | 70 |
| 11e | 2-Chloropyridine | −41 | 64 |
| 12f | 2-Chloropyridine | −41 | 85 |
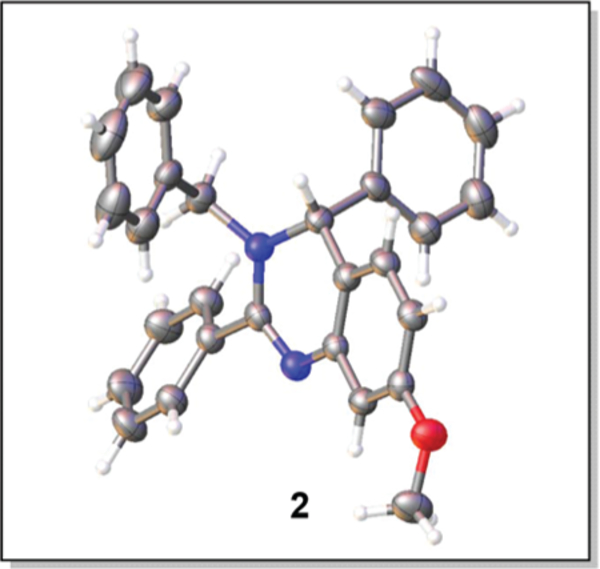
Conditions: 1 (1.0 mmol), benzylamine (1.1 mmol), benzaldehyde(1.1 mmol), 4 Å mol sieves (1.0 g), CH2Cl2 (10.0 mL), rt, 18 h; then base (1.2 mmol), Tf2O (1.1 mmol), temperature; then rt, 24 h.
Isolated yield.
4 Å mol sieves were not added.
N-Benzylidenebenzylamine (1.1 mmol) was used instead of benzylamine and benzaldehyde.
Reflux during final step.
48 h for final step.
