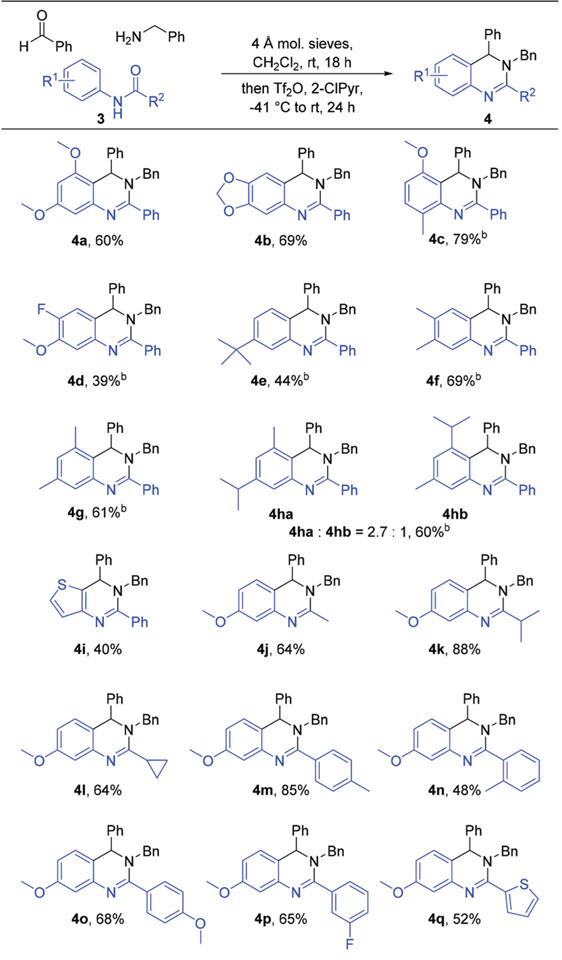
Table 2.
Synthesis of 3,4-dihydroquinazolines through variation of the starting amidea
Conditions: 3 (1.0 mmol), benzylamine (1.1 mmol), benzaldehyde (1.1 mmol), 4 Å mol sieves (1.0 g), CH2Cl2 (10.0 mL), rt, 18 h; then 2-ClPyr (1.2 mmol), Tf2O (1.1 mmol), −41 °C; then rt, 24 h. Isolated yield.
48 h for final step.