Abstract
Background:
The objective of this study was to compare fatigue levels between subjects with and without COPD, and to investigate the relationship between fatigue, demographics, clinical features and disease severity.
Methods:
A total of 1290 patients with COPD [age 65 ± 9 years, 61% male, forced expiratory volume in 1 s (FEV1) 56 ± 19% predicted] and 199 subjects without COPD (age 63 ± 9 years, 51% male, FEV1 112 ± 21% predicted) were assessed for fatigue (Checklist Individual Strength-Fatigue), demographics, clinical features and disease severity.
Results:
Patients with COPD had a higher mean fatigue score, and a higher proportion of severe fatigue (CIS-Fatigue score 35 ± 12 versus 21 ± 11 points, p < 0.001; 49 versus 10%, p < 0.001). Fatigue was significantly, but poorly, associated with the degree of airflow limitation [FEV1 (% predicted) Spearman correlation coefficient = −0.08, p = 0.006]. Multiple regression indicated that 30% of the variance in fatigue was explained by the predictor variables.
Conclusions:
Severe fatigue is prevalent in half of the patients with COPD, and correlates poorly with the degree of airflow limitation. Future studies are needed to better understand the physical, psychological, behavioural, and systemic factors that precipitate or perpetuate fatigue in COPD.
Keywords: airway obstruction, chronic obstructive pulmonary disease, fatigue
Introduction
Fatigue, defined as the subjective feeling of tiredness or exhaustion, is a common complaint in today’s society.1–5 It is a major distressing and persisting symptom in many chronic diseases, including Chronic Obstructive Pulmonary Disease (COPD).6 Fatigue is considered the second most important symptom of COPD after dyspnoea,7,8 and significantly impairs patients’ functional performance and quality of life (QoL).9–12 In patients with COPD, prevalence estimates of mild-to-severe fatigue range between 47% and 72%.7,8,13–17 Corresponding data from elderly, non-COPD subjects are limited. Most fatigue-related studies lack a non-COPD control group;7,8,15–17 and those studies that included a non-COPD control group report inconsistent results. Most of these studies indicate that the prevalence of fatigue is significantly higher in patients with COPD compared with elderly non-COPD subjects.13,14,18,19 Nevertheless, these studies were limited in size (between 30 and 37 control participants). One large population-based study, including 470 subjects with COPD and 659 without COPD, did not find a difference in fatigue level between patients with COPD and subjects without COPD.20
Despite the fact that fatigue is an important daily symptom in COPD, it is often ignored in clinical practice and research.11,21 Its aetiology is understudied and therefore subject to dispute, complicating the development of effective interventions to manage mild-to-severe fatigue in patients with COPD.22 Moreover, it remains unclear whether, and to what extent, fatigue is related to the lung function impairment itself. The association between fatigue and the degree of COPD severity has been studied before, but conclusions were contradictory.9,16,18,19,23,24 Based on the input of experts in the field of respiratory research and care, as well as patients with chronic lung disease, fatigue is prioritised as a research topic.25
A better understanding of the nature of fatigue and its underlying causes in patients with COPD will provide guidance for the development of pharmacological and nonpharmacological interventions for this important yet ignored symptom. Therefore, the objectives of this study were twofold: to compare fatigue levels between subjects with and without COPD; and to study the relationship between the severity of fatigue, demographic characteristics, clinical features and disease severity.
Methods
Study design and participants
The present study is a cross-sectional, comparative analysis combining baseline data from two prospective, observational studies and one randomized controlled trial [NL42721.060.12/M12-1280; P02.1411L CMO-nr 2002/047; and NCT00940355 (see clinicaltrials.gov)]. Details of these studies are published elsewhere.26,27 In addition, data from patients who were referred to a pulmonologist for a comprehensive health status assessment at the Amphia Hospital in Breda or the Radboud University Medical Centre in Nijmegen (both in the Netherlands) between April 2013 and June 2017, were conveniently used for the analyses.28–30 Patients were included in the current study if they had a diagnosis of COPD [Global initiative for chronic Obstructive Lung Disease (GOLD)],31 with a postbronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio, FEV1/FVC <0.7. This resulted in a total sample of 1290 outpatients with COPD from a secondary care setting.
The control group were all non-COPD participants from the two observational studies. In study NL42721.060.12/M12-1280, the resident proxy of the patient was recruited (defined as a person living together with a patient with COPD, regardless of whether they provide informal care to the patient with COPD). The recruitment area of this observational study concerned the southern-eastern part of the Netherlands. In study P02.1411L (CMO-nr 2002/047), healthy controls (individuals with no pre-existing medical conditions) were recruited by an advertisement in a regional newspaper (Nijmegen and surroundings, the Netherlands). The control groups of these studies were comparable in terms of marital status and working status but differed in educational level (16% versus 46%, respectively, completed secondary general education, p < 0.001). Non-COPD control subjects had no previous history of COPD and had a FEV1/FVC ratio greater than 0.7. In total, 199 non-COPD control subjects fulfilled the inclusion criteria and were selected for the current analysis.
The participating centres’ ethical review boards approved the two observational studies and one randomized controlled trial (NL42721.060.12/M12-1280; P02.1411L CMO-nr 2002/047; and NCT00940355). All participants in these studies provided written informed consent. With regard to the present analysis, consistent with the Dutch Central Committee on Research Involving Human Subjects guidance, reconsent for the processing of personal data was not required for subjects who have participated in approved studies within the scope of the Medical Research Involving Human Subjects Act (WMO). Furthermore, the Secretary of the Medical Ethics Committee of University Hospital Maastricht and Maastricht University (the medical ethical committee in our region) confirmed that additional ethics committee approval and informed consent were not needed for the present retrospective observational analysis. Data collected from patients in the Amphia Hospital in Breda or the Radboud University Medical Centre in Nijmegen were collected in the clinical routine and anonymized for research purposes according to local procedures. Due to the retrospective nature of the current analysis, and because the participants were subjected to usual care, the Medical Ethical Committee of the Radboudumc considered that it did not fall within the remit of the WMO, and, therefore, did not require informed consent nor additional ethics approval. Data of the participants from the Amphia Hospital in Breda or the Radboud University Medical Centre in Nijmegen were used in the study by Koolen and colleagues,30 and in the current analysis.
Measures
Fatigue was measured by the subjective fatigue subscale of the Checklist Individual Strength (CIS-Fatigue).32 The CIS-Fatigue consists of eight items scored on a seven-point Likert scale. The scores range from 8 (normal fatigue) to 56 (most severe fatigue). A score of 26 or lower indicates normal fatigue, scores between 27 and 35 indicate moderate fatigue and a score of 36 or higher indicates severe fatigue. The CIS-Fatigue is a standardized and validated questionnaire that has been used in healthy subjects,33–35 and among various patient populations, including COPD.16,27,32,36–38
Demographic data were collected from both subjects with COPD as well as controls without COPD, to provide information on their age, gender, working situation (yes/no paid job), marital status (yes/no partner), and education level (intermediate vocational education or lower/secondary general education or higher).
Clinical features included smoking status (yes/no current smoker), pack years [(number of cigarettes smoked per day/20) × number of years smoked], the number of medications in use, and the comorbidity burden [Charlson Comorbidity Index (CCI)].39 Body composition was assessed and expressed as body mass index (BMI). BMI was defined as the body mass in kilograms (kg) divided by the squared height in meters (kg/m2) and categorized in four subgroups: underweight (BMI < 21 kg/m2), normal weight (BMI 21–25 kg/m2), overweight (BMI 25–30 kg/m2), or obese (BMI > 30 kg/m2).40 The use of long-term oxygen therapy (yes/no) was assessed in patients only.
Disease severity was assessed by spirometry, the number of exacerbations in the previous year (patients only; defined as an acute event characterised by a worsening of the patient’s respiratory symptoms, that is, beyond the normal day-to-day variations and leads to a change in medication),41 the degree of dyspnoea [using the modified Medical Research Council (mMRC) scale] (patients only),42 and the disease-specific health status [COPD Assessment Test (CAT)] (patients only). Airflow obstruction was evaluated with postbronchodilator spirometry according to the ATS/ERS guidelines.43 Based on the degree of airflow limitation patients were classified according to the 2007 GOLD stages: GOLD grade 1 (FEV1 ⩾80% of predicted), GOLD grade 2 (50%⩽ FEV1 <80% of predicted), GOLD grade 3 (30%⩽ FEV1 <50% of predicted), GOLD grade 4 (FEV1 <30% of predicted).44
Statistical analyses
Data were analysed using SPSS (V.25.0 for Windows, Chicago, IL, USA). Categorical data were presented as frequencies and percentages. Continuous variables were tested for normality using the Shapiro–Wilk test, and presented as mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate. Differences between patients and non-COPD control subjects were analysed with the Chi-square test (categorical data), Student’s t test for independent groups (continuous data), or the Mann–Whitney U test (skewed continuous data). The Kruskall–Wallis test was administered to determine if there were differences between patients with normal, mild, or severe fatigue. A priori, the level of significance was set at p < 0.01 (two-tailed).
Correlations were assessed by the nonparametric Spearman’s rank correlation. The magnitude of the relationships was interpreted using the effect size provided by Cohen. Correlation coefficients in the order of 0.10 represent a small association, coefficients of 0.30 are a medium association, and coefficients of 0.50 represent a large effect size.45 Last, multiple regression analysis was performed to determine how much of the variation in the fatigue level could be explained by the predictor variables. The predictor variables were those that significantly correlated with fatigue (p < 0.10). Independent variables were checked for multicollinearity by inspecting the tolerance values/variance inflation factors (VIF) and the correlation coefficients of the independent variables. There is no evidence for multicollinearity when tolerance values are greater than 0.1 (which is a VIF less than 10) and correlation coefficients below 0.7. The CAT questionnaire was left out, since it was expected that the CAT would explain a large share of the variance of fatigue, considering that the CAT questionnaire includes a question on the participant’s energy level.
Results
Background characteristics
Table 1 presents the background characteristics of the 1290 COPD patients and the 199 non-COPD participants. The vast majority of patients with COPD had moderate-to-severe COPD (49% GOLD stage II, and 33% GOLD stage III). The groups were comparable in terms of age, proportion of current smokers, education, and working situation. The proportion of men in the COPD sample tended to be higher compared with the non-COPD control group (p = 0.011). Compared with the non-COPD control group, patients with COPD lived more often alone, had a worse lung function, had a lower BMI, had a higher number of comorbidities, and used more medications.
Table 1.
Background characteristics of COPD patients (n = 1290) and non-COPD participants (n = 199).
| General characteristics | n | COPD (n = 1290) | n | Non-COPD (n = 199) | p-value |
|---|---|---|---|---|---|
| Male, n (%) | 1290 | 784 (60.8) | 199 | 102 (51.3) | 0.011 |
| Age, years | 1290 | 64.7 ± 9.4 | 199 | 63.1 ± 9.4 | 0.053 |
| Current smoker, n (%) | 1232a | 380 (30.8) | 199 | 45 (22.6) | 0.018 |
| Pack years | 194b | 40.3 ± 20 | 133l | 16.8 ± 20.6 | <0.001 |
| Long term oxygen therapy, n (%) | 194c | 54 (27.8) | - | - | - |
| Charlson comorbidity index, points | 543d | 2 (1–3) | 66m | 1 (0–2) | <0.001 |
| COPD assessment test, points | 696e | 19 (13–25) | - | - | - |
| Partner, n (%) | 1250f | 951 (76.1) | 199 | 169 (84.9) | 0.006 |
| Level of education, n (%) | 1226g | 199 | 0.585 | ||
| Intermediate vocational education or lower | 889 (72.5) | 148 (74.4) | |||
| Secondary general education or higher | 337 (27.5) | 51 (25.6) | |||
| Working status, n (%) | 1237h | 198n | 0.415 | ||
| Paid work | 310 (25.1) | 55 (27.8) | |||
| No paid work | 927 (74.9) | 143 (72.2) | |||
| Body composition | |||||
| BMI (kg/m2) | 1290 | 26 ± 5 | 199 | 27.5 ± 4.5 | <0.001 |
| Underweight, n (%) | 203 (15.7) | 9 (4.5) | <0.001 | ||
| Normal weight, n (%) | 384 (29.8) | 58 (29.1) | |||
| Overweight, n (%) | 455 (35.3) | 82 (41.2) | |||
| Obese, n (%) | 248 (19.2) | 50 (25.1) | |||
| Spirometry | |||||
| FEV 1 (% predicted) | 1290 | 55.5 ± 18.9 | 199 | 111.5 ± 20.8 | <0.001 |
| FEV 1 (Litres) |
1290 | 1.6 ± 0.6 | 199 | 3.1 ± 0.8 | <0.001 |
| FVC (Litres) | 1290 | 3.5 ± 1 | 199 | 4 ± 1.1 | <0.001 |
| GOLD, n (%) | 1290 | - | |||
| I | 131 (10.2) | - | - | ||
| II | 631 (48.9) | - | - | ||
| III | 430 (33.3) | - | - | ||
| IV | 98 (7.6) | - | - | ||
| Medications in use, nr | 194i | 7 (5–11) | 133o | 2 (1–5) | <0.001 |
| Dyspnoea | |||||
| mMRC, points | 744j | 2(1–3) | - | ||
| <2 mild dyspnoea, n (%) | 346 (46.5) | - | - | - | |
| ⩾2 severe dyspnoea, n (%) | 398 (53.5) | - | - | ||
| Fatigue | |||||
| CIS-Fatigue, points | 1290 | 35 (26–44) | 199 | 20 (12–28) | <0.001 |
| Normal fatigue, n (%) | 329 (25.5) | 146 (73.4) | <0.001 | ||
| Mild fatigue, n (%) | 335 (26.0) | 33 (16.6) | |||
| Severe fatigue, n (%) | 626 (48.5) | 20 (10.1) | |||
| Exacerbations last 12 months, n | 713k | 1 (0–2) | - | - | - |
Data are shown as mean ± SD, median (IQR) or n(%).
BMI, body mass index; CIS-Fatigue, checklist individual strength subscale fatigue; COPD, chronic obstructive pulmonary disease; FEV1 (% predicted), forced expiratory volume over 1 s of predicted; FVC, forced volume capacity; GOLD, global initiative for chronic obstructive lung disease; IQR, interquartile range; mMRC, modified Medical Research Council dyspnoea scale; SD, standard deviation.
58 missing values; b1096 missing values; c1096 missing values; d747 missing values; e594 missing values; f40 missing values; g64 missing values; h53 missing values; i1096 missing values; j546 missing values; k572 missing values; l66 missing values; m133 missing values; n1 missing values; o66 missing values.
Fatigue
Among patients with COPD, 25.5% experienced normal fatigue, 26.0% mild fatigue, and 48.5% severe fatigue. In comparison, 73.4% of the non-COPD subjects reported normal fatigue, 16.6% mild fatigue, and 10.1% severe fatigue (p < 0.001; Figure 1). Moreover, mean fatigue scores were significantly higher in the COPD group versus the non-COPD group (Table 1). Compared with male patients with COPD, female patients had a higher mean fatigue score, and, in turn, a higher proportion of severe fatigue (CIS-Fatigue score 34 ± 12 versus 37 ± 12 points, p < 0.001; 45 versus 54% with severe fatigue, respectively; p < 0.001; see Table S1). The mean fatigue score of the women and men in the non-COPD control group were comparable (22 ± 12 versus 21 ± 11 points, respectively; p = 0.39; see Table S2). Within the non-COPD controls, the controls from the NL42721.060.12/M12-1280 study had a higher mean fatigue score compared with the control group selected from the P02.1411L CMO-nr 2002/047 study (CIS-Fatigue score 24 ± 12 versus 16 ± 8 points; p < 0.001; see Table S3).
Figure 1.
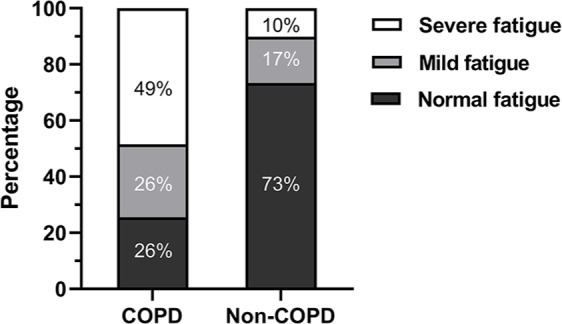
Prevalence of normal, mild and severe fatigue in persons with and without COPD.
COPD, chronic obstructive pulmonary disease.
Differences among patients with normal, mild or severe fatigue
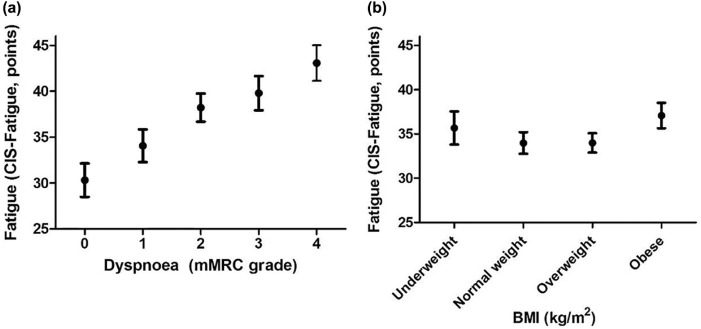
Table 2 lists the characteristics of patients with COPD stratified by the degree of fatigue. Most notably, patients with severe fatigue had greater dyspnoea (mMRC grade ⩾2), compared with patients with normal and mild fatigue (normal fatigue: 28%; mild fatigue: 49%; severe fatigue: 65%; p < 0.001; Figure 2a). Moreover, the patients who experienced severe fatigue were somewhat younger, had a worse lung function, were more often smokers, used a higher number of medications, and experienced more exacerbations in the last 12 months. Disease-specific health status, measured with the CAT, was more impaired in patients with severe fatigue compared with subjects with normal and mild fatigue. The groups were comparable in terms of pack years, marital status, the number of patients that used oxygen therapy, self-reported comorbidities, education level, working status, and BMI. Patients categorized as being underweight as well as obese experienced more severe fatigue than patients classified as having a normal or overweight BMI (Figure 2b).
Table 2.
Characteristics of COPD patients with normal, mild or severe fatigue.
| Fatigue level – COPD patients | n | Normal fatigue (n = 329) | n | Mild fatigue (n = 335) | n | Severe fatigue (n = 626) | p-value |
|---|---|---|---|---|---|---|---|
| Male, n (%) | 329 | 225(68.4) | 335 | 208(62.1) | 626 | 351(56.1) | 0.001 |
| Age, years | 329 | 65.4 ± 8.7 | 335 | 65.6 ± 9.4 | 626 | 63.9 ± 9.8 | 0.006 |
| Current smoker, n (%) | 318a | 88(27.7) | 312l | 71(22.8) | 602x | 221(36.7) | <0.001 |
| Pack years | 35b | 37 ± 18.3 | 57m | 38.3 ± 19.4 | 102y | 42.5 ± 20.7 | 0.300 |
| Long term oxygen therapy, n (%) | 35b | 6(17.1) | 57n | 15(26.3) | 102z | 33(32.4) | 0.213 |
| Charlson comorbidity index, points | 113d | 2(1–3) | 129o | 2(1–3) | 301aa | 2(1–4) | 0.114 |
| COPD Assessment Test, points | 144e | 11(8–14) | 171p | 17(12–21) | 381ab | 22(18–27) | <0.001 |
| Partner, n (%) | 323f | 258(79.9) | 329q | 257(78.1) | 598ac | 436(72.9) | 0.037 |
| Level of education, n (%) | 325g | 320r | 581ad | 0.076 | |||
| Intermediate vocational education or lower | 220(67.7) | 237(74.1) | 432(74.4) | ||||
| Secondary general education or higher | 105(32.3) | 83(25.9) | 149(25.6) | ||||
| Working status, n (%) | 322h | 323s | 592ae | 0.548 | |||
| Paid work | 88(27.3) | 79(24.5) | 143(24.2) | ||||
| No Paid work | 234(72.7) | 244(75.5) | 449(75.8) | ||||
| Body composition | |||||||
| BMI (kg/m2) | 329 | 25.6 ± 4.3 | 335 | 25.9 ± 4.6 | 626 | 26.3 ± 5.6 | 0.390 |
| Underweight, n (%) | 53(16.1) | 335 | 40(11.9) | 626 | 110(17.6) | 0.002 | |
| Normal weight, n (%) | 104(31.6) | 110(32.8) | 170(27.2) | ||||
| Overweight, n (%) | 123(37.4) | 131(39.1) | 201(32.1) | ||||
| Obese, n (%) | 49(14.9) | 54(16.1) | 145(23.2) | ||||
| Spirometry | |||||||
| FEV 1 (% predicted) | 329 | 59.1 ± 19 | 335 | 54.8 ± 19.4 | 626 | 54.1 ± 18.3 | 0.001 |
| FEV 1 (Litres) | 329 | 1.7 ± 0.6 | 1.5 ± 0.7 | 1.5 ± 0.6 | <0.001 | ||
| FVC (Litres) | 329 | 3.7 ± 1 | 3.4 ± 1 | 3.4 ± 1 | <0.001 | ||
| GOLD, n (%) | 329 | 335 | 626 | 0.005 | |||
| I | 44(13.4) | 38(11.3) | 49(7.8) | ||||
| II | 173(52.6) | 152(45.4) | 306(48.9) | ||||
| III | 98(29.8) | 111(33.1) | 221(35.3) | ||||
| IV | 14(4.3) | 34(10.1) | 50(8.0) | ||||
| Medications in use, n | 35i | 6(3–9) | 57t | 7(4–9.5) | 102af | 8(6–13) | 0.001 |
| Dyspnoea | |||||||
| mMRC, points | 149j | 1(0–2) | 192u | 1(0–3) | 403ag | 2(1–3) | <0.001 |
| <2 mild dyspnoea, n (%) | 107(71.8) | 192v | 98(51.0) | 403ah | 141(35.0) | <0.001 | |
| ⩾2 severe dyspnoea, n (%) | 42(28.2) | 94(49.0) | 262(65.0) | ||||
| Exacerbations last 12 months, n | 149k | 0(0–1) | 183w | 1(0–2) | 381ai | 1(0–2) | <0.001 |
Data are shown as mean ± SD, median (IQR) or n(%). p-value: Kruskall–Wallis comparing patients with normal, mild and severe fatigue.
BMI, body mass index; CIS-Fatigue, checklist individual strength subscale fatigue; COPD, chronic obstructive pulmonary disease; FEV1 (% predicted), forced expiratory volume over 1 s of predicted; FVC, forced volume capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IQR, interquartile range; mMRC, modified Medical Research Council dyspnoea scale; SD, standard deviation.
11 missing values; b294 missing values; c294 missing values; d216 missing values; e185 missing values; f6 missing values; g4 missing values; h7 missing values; i294 missing values; j180 missing values; k180 missing values; l23 missing values; m278 missing values; n278 missing values; o206 missing values; p164 missing values; q6 missing values; r21 missing values; s12missing values; t278 missing values; u421 missing values; v143 missing values; w152 missing values; x24 missing values; y524 missing values; z524 missing values; aa325 missing values; ab245 missing values; ac28 missing values; ad45 missing values; ae34 missing values; af524 missing values; ag223 missing values; ah223 missing values; ai245 missing values.
Figure 2.
(a) Fatigue scores (mean and 95% CI) after stratification for the degree of dyspnoea: grade 0–4 on the mMRC dyspnoea scale. (b) Fatigue scores (mean and 95% CI) after stratification for the BMI classification (underweight BMI < 20 kg/m2, normal weight BMI 20–25 kg/m2, overweight BMI 25–30 kg/m2, obese BMI > 30 kg/m2).
BMI, body mass index; CI, confidence interval; mMRC, modified Medical Research Council.
Factors associated with fatigue in patients with COPD
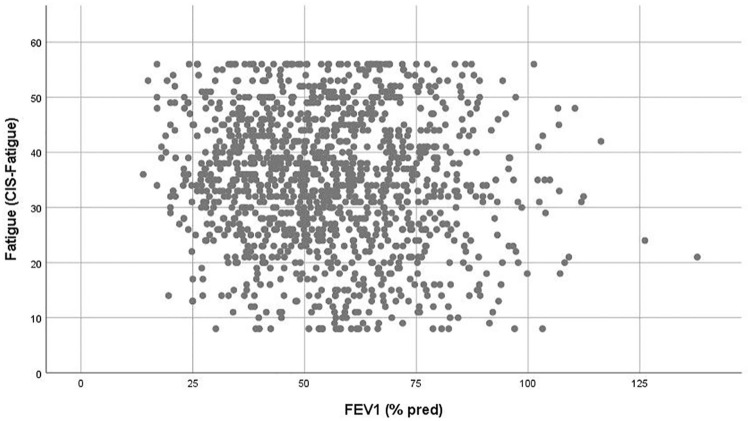
Education level, work situation, and BMI showed no significant relation with fatigue (p > 0.10). The factors gender (rs = 0.113, p < 0.001), age (rs = −0.087, p = 0.002), GOLD grade (rs = 0.076, p = 0.006), marital status (rs = −0.096, p = 0.001), smoking status (rs = 0.127, p < 0.001), pack years (rs = 0.129, p = 0.073), the use of long-term oxygen therapy (rs = 0.146, p = 0.043), comorbidity burden (rs = 0.104, p = 0.016), number of COPD-related exacerbations in the previous year (rs = 0.199, p < 0.001), and lung function (FEV1 (L) rs = −0.085, p = 0.002; FEV1 (% predicted) rs = −0.076, p = 0.006; FVC (L) rs = −0.143, p < 0.001) (Figure 3) showed significant but low associations with fatigue (p < 0.10). The number of medications (rs = 0.353, p < 0.001) and the degree of dyspnoea (rs = 0.347, p < 0.001) showed the strongest relation with fatigue. Moreover, as expected, fatigue was strongly correlated with the CAT questionnaire (rs = 0.584, p < 0.001) (Figure 4).
Figure 3.
Scatterplot showing the poor association between fatigue and FEV1 (% predicted).
FEV1, forced expiratory volume over 1 s of predicted.
Figure 4.
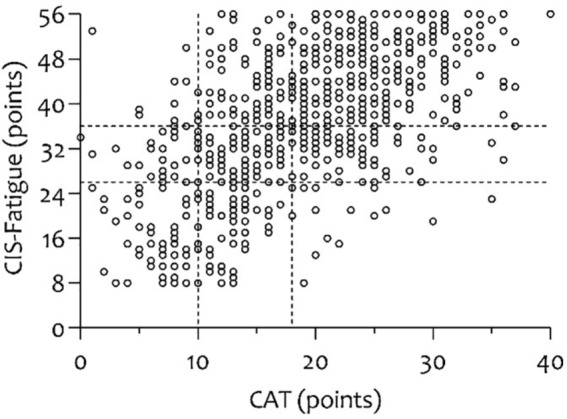
Scatterplot showing the strong association between fatigue and the CAT.
CAT, COPD assessment test
R2 for the overall model was 29.7%. Gender, age, marital status, smoking status, pack years, FEV1 (% predicted), FVC (L), the use of long-term oxygen therapy, number of medications, the comorbidity burden (CCI), the degree of dyspnoea (mMRC), and the number of exacerbations in the previous year significantly predicted fatigue in COPD, F(12, 176) = 7.630 (p < 0.001; in which 12 indicates the regression degrees of freedom, 176 the residual degrees of freedom, 7.630 the obtained F value). The variable ‘GOLD stadia’ was excluded from the multiple regression, since it was highly intercorrelated with the variable ‘FEV 1 (% predicted)’ (bivariate correlation coefficient of −0.91, respectively), and therefore reflects the same concept, that is, airflow limitation. Of all independent variables, dyspnoea as measured by the mMRC scale was responsible for the largest unique contribution (Beta = 0.32, p < 0.001) (Table S4).
Discussion
The most significant result of this study is that severe fatigue is more prevalent in patients with COPD compared with subjects without COPD. Furthermore, fatigue appears to be significantly, but poorly associated with the degree of airflow limitation. Moreover, 70% of the variance in fatigue scores cannot be explained by demographics, clinical features and disease severity.
Prevalence of fatigue
A quarter of patients with COPD experienced mild fatigue, whereas half had severe fatigue. In comparison, 17% of subjects without COPD experienced mild fatigue and only 10% had severe fatigue. These results indicate that severe fatigue is substantially more prevalent in patients with COPD compared with elderly without COPD (p < 0.001). Despite its high prevalence and significant negative health consequences,9–12 fatigue remains often undiagnosed, and, in turn, untreated.11,21 Therefore, the prevalence emphasizes the clinical relevance of assessing fatigue in patients with COPD. Nevertheless, a better understanding of the factors that precipitate or perpetuate fatigue in patients with COPD is needed, to develop fatigue reducing or coping interventions for this important, yet disregarded, symptom in patients with COPD.22,46
The current findings on the prevalence of fatigue in patients with COPD and non-COPD participants are in line with previous studies,13,14,18,19 although, the control groups of those studies were not only limited in size, they were not always well characterized. For example, Theander and colleagues reported fatigue every day during the preceding month in nearly half of the patients, as measured with the Fatigue Impact Scale (FIS), compared with 13.5% of the control group (p < 0.001)14 However, lung function was not assessed in either the COPD or the control group. To date, one population-based study by Andersson and colleagues has been published that included a large non-COPD control group (470 subjects with COPD and 659 without COPD, respectively).20 However, this study did not find a difference in fatigue level between patients with COPD and subjects without COPD. This might be attributed to the fact that the included patients with COPD had a lower disease severity compared with the patients in the current analysis (FEV1 86% versus 56% of predicted, 33% versus 54% had a dyspnoea score ⩾2). Nevertheless, we also showed a significant, but poor, correlation between fatigue and airflow limitation. Lastly, the current findings on the proportion of patients with severe fatigue (49%) are in accordance with prevalence estimates of other airway diseases such as asthma (63%).38
Factors associated with fatigue in patients with COPD
There were no clear associations identified between fatigue and demographic characteristics, clinical features and disease severity. In particular, a significant but poor correlation was found between fatigue and the degree of airflow limitation. This finding is in contrast with that of a previous study by Breslin and colleagues, who showed a significantly negative correlation between fatigue and FEV1 % predicted (r = −0.32; p < 0.05).24 The current findings are more in line with recent studies by Kapella and colleagues, Baghai-Ravary and colleagues, and Lewko and colleagues.9,18,19 Moreover, a longitudinal study on 77 patients with COPD showed that the lung function did not worsen during 4-year follow-up, while the proportion of patients with COPD with mild-to-severe fatigue doubled.16 These results strongly suggest that the degree of airflow limitation may not be the primary underlying cause of fatigue in patients with COPD.
The only moderate correlations found between demographic characteristics, clinical features, disease severity and fatigue concerned dyspnoea (rs = 0.35, p < 0.001) and the number of medications (rs = 0.35, p < 0.001). As expected, a significant association was found between dyspnoea and fatigue, since these sensations are often reported to concur.9,15,47–49 Nevertheless, Reihstein and colleagues observed a correlation between fatigue and dyspnoea of a moderate magnitude (r = 0.43), similar to this study,47 whereas studies by Gift and Shepard (r = 0.63), Woo and colleagues (r = 0.69), Kapella and colleagues (r = 0.74) and Kinsman and colleagues (r = 0.76) found a correlation of a large magnitude between fatigue and dyspnoea.9,15,48,49 The use of different measures to assess fatigue severity as well as dyspnoea severity might have influenced these results. This is the first study to find a significant positive correlation between the number of medications used and fatigue. The role of pulmonary medication in the treatment of fatigue is probably limited since the degree of airflow limitation is poorly associated with the fatigue. A causal relationship cannot be established based on the current cross-sectional data. Therefore, it remains unknown whether, and to what extent, this observation may be due to side effects of medication used, or may be caused by the conditions for which the medications were prescribed. Future COPD drug trials may need to monitor fatigue as a possible adverse symptom.
Explained variance of fatigue in COPD
Although 30% of the variance in fatigue scores was explained by the demographic characteristics, clinical features and disease severity, 70% of the variance remained unexplained in the present study. This indicates that fatigue is a complex symptom and the causes should be sought not only in conventional demographic characteristics, clinical features and disease characteristics only, but also rather in a combination of physical, psychological, systemic and behavioural factors (precipitating and perpetuating factors).46
Strengths and limitations
Some limitations should be acknowledged. First, 80% of the patients with COPD were classified as GOLD II and III, indicating that the current results cannot be generalized to patients with mild or very severe COPD. Second, the included patients with COPD were recruited from a secondary care setting. Therefore, results cannot be generalized to primary and tertiary care. Third, due to the retrospective nature of the analyses, it was not possible to retrieve information on the specific medication that was used by the participants, which could have given us insights into the relation between pulmonary medication and fatigue. Moreover, it should be noted that for some variables (e.g. pack years, use of long-term oxygen therapy, and the number of medications), not all data were available. For this reason, the multiple regression model is based on data from 189 patients. Despite the smaller sample size, the results of the multiple regression indicate that the causes of fatigue cannot be explained only by demographics, clinical features and disease severity. Factors such as depressive symptoms may potentially also contribute to the explanation of fatigue in patients with COPD.12,13,18,50,51 Nevertheless, it may be difficult to distinguish if fatigue is a symptom of the depression itself, or emerges as an adverse effect of antidepressant drugs. Besides depressive symptoms, patients with COPD also often report poor sleep quality.13 Moreover, patients with COPD and sleep apnoea (overlap syndrome patients) express significantly more fatigue than sleep apnoeic patients only.52 Therefore, undetected sleep apnoea and poor sleep quality should be considered in future studies. In addition, factors such as physical inactivity and kinesiophobia,20,53 but also other comorbidities like concomitant heart disease have been reported to be associated with fatigue in COPD and should be further explored.54 Low-grade systemic inflammation has also been observed in patients with clinically stable COPD.55 Nevertheless, to date, little research has been done to investigate which inflammatory markers are possibly related to fatigue in COPD.56,57 Fourth, because of the cross-sectional design, we were not able to examine whether the associations between fatigue and the conventional COPD characteristics were temporary or fluctuate over time. In light of this, the use of Ecological Momentary Assessment (EMA) would have provided us with more insight into the day-to-day variability of fatigue.46,58,59 Fifth, the direct impact of an exacerbation and, in particular, exacerbation-related hospitalization on fatigue could not be evaluated. A longitudinal study design in which additional measurements are conducted during and after an exacerbation-related hospitalization would provide us with more information on the impact of an exacerbation on fatigue.46 At last, information on the impact of fatigue on QoL and the relationship with commonly reported symptoms in COPD (such as cough and sputum) should be described in future studies.46
Strengths of the current study are the large patient sample size as well as the inclusion of 199 non-COPD control subjects with measured lung function. The differences in the cohorts increases the findings’ external validity and likelihood that the results are representative for the entire COPD population (Tables S3 and S5). The additional use of the validated CIS-Fatigue checklist enables the calculation of the prevalence of fatigue and the discrimination between normal, mild and severe fatigue.
Conclusion
This study corroborates earlier findings and demonstrates that severe fatigue is highly prevalent and more common in patients with COPD compared with non-COPD control subjects, and correlates poorly with the degree of airflow limitation. These findings emphasize the clinical importance of assessing fatigue in patients with COPD. Moreover, the causes of fatigue in COPD should no longer be sought only in the demographic characteristics, clinical features, and disease severity. Future studies should focus on the influence of physical, psychological, behavioural, and systemic factors that could potentially precipitate or perpetuate fatigue in patients with COPD.
Supplemental Material
Supplemental material, Revision_Online_supplement_FatigueCOPD_Ther_Adv_Resp_Dis_YMJGoertz_3April2019 for Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation by Yvonne M. J. Goërtz, Martijn A. Spruit, Alex J. Van ‘t Hul, Jeannette B. Peters, Maarten Van Herck, Nienke Nakken, Remco S. Djamin, Chris Burtin, Melissa S. Y. Thong, Arnold Coors, Yvonne Meertens-Kerris, Emiel F. M. Wouters, Judith B. Prins, Frits M. E. Franssen, Jean W. M. Muris, Lowie E. G. W. Vanfleteren, Mirjam A. G. Sprangers, Daisy J. A. Janssen and Jan H. Vercoulen in Therapeutic Advances in Respiratory Disease
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This project is supported by the Lung foundation Netherlands, Leusden, the Netherlands (grant numbers 4.1.16.085 and 3.4.12.024); AstraZeneca, the Netherlands; Boehringer Ingelheim, the Netherlands; Stichting Astma Bestrijding, the Netherlands; Hasselt University, Belgium; and CIRO, the Netherlands.
Conflict of interest statement: AstraZeneca, the Netherlands; Boehringer Ingelheim, the Netherlands
ORCID iD: Yvonne M. J. Goërtz  https://orcid.org/0000-0003-4674-9562
https://orcid.org/0000-0003-4674-9562
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yvonne M. J. Goërtz, Department of Research and Education, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, NM 6085, the Netherlands.
Martijn A. Spruit, Department of Research and Education, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, NM, the Netherlands REVAL - Rehabilitation Research Center, BIOMED - Biomedical Research Institute, Faculty of Rehabilitation Sciences, Hasselt University, Diepenbeek, Belgium; Department of Respiratory Medicine, Maastricht University Medical Centre (MUMC+), Maastricht, the Netherlands; NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht, the Netherlands.
Alex J. Van ‘t Hul, Department of Pulmonary Disease, Radboud University Medical Center, Nijmegen, the Netherlands
Jeannette B. Peters, Department of Pulmonary Disease, Radboud University Medical Center, Nijmegen, the Netherlands Department of Medical Psychology, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
Maarten Van Herck, REVAL - Rehabilitation Research Center, BIOMED - Biomedical Research Institute, Faculty of Rehabilitation Sciences, Hasselt University, Diepenbeek, Belgium.
Nienke Nakken, Department of Research and Education, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, the Netherlands.
Remco S. Djamin, Department of Respiratory Medicine, Amphia Ziekenhuis, Breda, the Netherlands
Chris Burtin, REVAL - Rehabilitation Research Center, BIOMED - Biomedical Research Institute, Faculty of Rehabilitation Sciences, Hasselt University, Diepenbeek, Belgium.
Melissa S. Y. Thong, Department of Medical Psychology, Amsterdam University Medical Centres, location AMC, Amsterdam, the Netherlands
Arnold Coors, Member of the Patient Advisory Board, Radboud University Medical Center, Nijmegen, the Netherlands.
Yvonne Meertens-Kerris, Member of the Patient Advisory Board, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, the Netherlands.
Emiel F. M. Wouters, Department of Research and Education, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, the Netherlands Department of Respiratory Medicine, Maastricht University Medical Centre (MUMC+), Maastricht, the Netherlands.
Judith B. Prins, Department of Medical Psychology, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands
Frits M. E. Franssen, Department of Research and Education, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, the Netherlands Department of Respiratory Medicine, Maastricht University Medical Centre (MUMC+), Maastricht, the Netherlands; NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht, the Netherlands.
Jean W. M. Muris, Department of Family Medicine, CAPHRI Care and Public Health Research Institute, Maastricht University, Maastricht, the Netherlands
Lowie E. G. W. Vanfleteren, Department of Research and Education, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, the Netherlands Department of Respiratory Medicine, Maastricht University Medical Centre (MUMC+), Maastricht, the Netherlands; COPD Center, Sahlgrenska University, Gothenburg, Sweden.
Mirjam A. G. Sprangers, Department of Medical Psychology, Amsterdam University Medical Centres, location AMC, Amsterdam, the Netherlands
Daisy J. A. Janssen, Department of Research and Education, Ciro, Centre of Expertise for Chronic Organ Failure, Horn, the Netherlands Centre of Expertise for Palliative Care, Maastricht University Medical Centre (MUMC+), Maastricht, the Netherlands.
Jan H. Vercoulen, Department of Pulmonary Disease, Radboud University Medical Center, Nijmegen, the Netherlands Department of Medical Psychology, Radboud University Medical Center, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
References
- 1. Bultmann U, Kant I, Kasl SV, et al. Fatigue and psychological distress in the working population: psychometrics, prevalence, and correlates. J Psychosom Res 2002; 52: 445–452. [DOI] [PubMed] [Google Scholar]
- 2. Chen MK. The epidemiology of self-perceived fatigue among adults. Prev Med 1986; 15: 74–81. [DOI] [PubMed] [Google Scholar]
- 3. Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res 1998; 45: 53–65. [DOI] [PubMed] [Google Scholar]
- 4. Pawlikowska T, Chalder T, Hirsch SR, et al. Population based study of fatigue and psychological distress. BMJ 1994; 308: 763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ream E, Richardson A. Fatigue in patients with cancer and chronic obstructive airways disease: a phenomenological enquiry. Int J Nurs Stud 1997; 34: 44–53. [DOI] [PubMed] [Google Scholar]
- 6. Janson-Bjerklie S, Carrieri VK, Hudes M. The sensations of pulmonary dyspnea. Nurs Res 1986; 35: 154–159. [PubMed] [Google Scholar]
- 7. Blinderman CD, Homel P, Billings JA, et al. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J Pain Symptom Manag 2009; 38: 115–123. [DOI] [PubMed] [Google Scholar]
- 8. Walke LM, Byers AL, Tinetti ME, et al. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Archiv Intern Med 2007; 167: 2503–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapella MC, Larson JL, Patel MK, et al. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nurs Res 2006; 55: 10–17. [DOI] [PubMed] [Google Scholar]
- 10. Stridsman C, Skar L, Hedman L, et al. Fatigue affects health status and predicts mortality among subjects with COPD: report from the population-based OLIN COPD study. COPD 2015; 12: 199–206. [DOI] [PubMed] [Google Scholar]
- 11. Kouijzer M, Brusse-Keizer M, Bode C. COPD-related fatigue: impact on daily life and treatment opportunities from the patient’s perspective. Respir Med 2018; 141: 47–51. [DOI] [PubMed] [Google Scholar]
- 12. Stridsman C, Svensson M, Strandkvist VJ, et al. The COPD Assessment Test (CAT) can screen for fatigue among patients with COPD. Ther Adv Respir Dis 2018; 12: L1–L10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kentson M, Todt K, Skargren E, et al. Factors associated with experience of fatigue, and functional limitations due to fatigue in patients with stable COPD. Ther Adv Respir Dis 2016; 10: 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theander K, Unosson M. Fatigue in patients with chronic obstructive pulmonary disease. J Adv Nurs 2004; 45: 172–177. [DOI] [PubMed] [Google Scholar]
- 15. Gift AG, Shepard CE. Fatigue and other symptoms in patients with chronic obstructive pulmonary disease: do women and men differ? JOGNN 1999; 28: 201–208. [DOI] [PubMed] [Google Scholar]
- 16. Peters JB, Heijdra YF, Daudey L, et al. Course of normal and abnormal fatigue in patients with chronic obstructive pulmonary disease, and its relationship with domains of health status. Patient Educ Couns 2011; 85: 281–285. [DOI] [PubMed] [Google Scholar]
- 17. Inal-Ince D, Savci S, Saglam M, et al. Fatigue and multidimensional disease severity in chronic obstructive pulmonary disease. Multidiscip Respir Med 2010; 5: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baghai-Ravary R, Quint JK, Goldring JJ, et al. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med 2009; 103: 216–223. [DOI] [PubMed] [Google Scholar]
- 19. Lewko A, Bidgood PL, Garrod R. Evaluation of psychological and physiological predictors of fatigue in patients with COPD. BMC Pulm Med 2009; 9: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersson M, Stridsman C, Ronmark E, et al. Physical activity and fatigue in chronic obstructive pulmonary disease: a population based study. Respir Med 2015; 109: 1048–1057. [DOI] [PubMed] [Google Scholar]
- 21. Janssen DJ, Spruit MA, Uszko-Lencer NH, et al. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med 2011; 14: 735–743. [DOI] [PubMed] [Google Scholar]
- 22. Spruit MA, Vercoulen JH, Sprangers MAG, et al. Fatigue in COPD: an important yet ignored symptom. Lancet Respir Med 2017; 5: 542–544. [DOI] [PubMed] [Google Scholar]
- 23. Breukink SO, Strijbos JH, Koorn M, et al. Relationship between subjective fatigue and physiological variables in patients with chronic obstructive pulmonary disease. Respir Med 1998; 92: 676–682. [DOI] [PubMed] [Google Scholar]
- 24. Breslin E, van der Schans C, Breukink S, et al. Perception of fatigue and quality of life in patients with COPD. Chest 1998; 114: 958–964. [DOI] [PubMed] [Google Scholar]
- 25. Postma DS, Wijkstra PJ, Hiemstra PS, et al. The Dutch National Program for Respiratory Research. Lancet Respir Med 2016; 4: 356–357. [DOI] [PubMed] [Google Scholar]
- 26. Nakken N, Janssen DJ, van den Bogaart EH, et al. An observational, longitudinal study on the home environment of people with chronic obstructive pulmonary disease: the research protocol of the Home Sweet Home study. BMJ Open 2014; 4: e006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vercoulen JH, Daudey L, Molema J, et al. An Integral assessment framework of health status in chronic obstructive pulmonary disease (COPD). Int J Behav Med 2008; 15: 263–279. [DOI] [PubMed] [Google Scholar]
- 28. Van den Akker EF, Van‘t Hul AJ, Chavannes NH, et al. Development of an integral assessment approach of health status in patients with obstructive airway diseases: the CORONA study. Int J Chron Obstruct Pulmon Dis 2015; 10: 2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smid DE, Spruit MA, Deeg DJH, et al. How to determine an impaired health status in COPD: Results from a population-based study. Neth J Med 2017; 75: 151–157. [PubMed] [Google Scholar]
- 30. Koolen EH, Van Hees HW, Van Lummel RC, et al. ‘Can do’ versus ‘do do’: a novel concept to better understand physical functioning in patients with chronic obstructive pulmonary disease. J Clin Med 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Global Initiative for Chronic Obstructive Lung Disease. From the Global Strategy fort he diagnosis, management and prevention of COPD, http://www.goldcopd.org/ (accessed 27 October 2017).
- 32. Vercoulen JH, Swanink CM, Fennis JF, et al. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 1994; 38: 383–392. [DOI] [PubMed] [Google Scholar]
- 33. Beurskens AJ, Bultmann U, Kant I, et al. Fatigue among working people: validity of a questionnaire measure. Occup Environ Med 2000; 57: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bultmann U, De Vries M, Beurskens AJ, et al. Measurement of prolonged fatigue in the working population: determination of a cutoff point for the checklist individual strength. J Occup Health Psychol 2000; 5: 411–416. [DOI] [PubMed] [Google Scholar]
- 35. Worm-Smeitink M, Gielissen M, Bloot L, et al. The assessment of fatigue: psychometric qualities and norms for the Checklist individual strength. J Psychosom Res 2017; 98: 40–46. [DOI] [PubMed] [Google Scholar]
- 36. Repping-Wuts H, Fransen J, van Achterberg T, et al. Persistent severe fatigue in patients with rheumatoid arthritis. J Clin Nurs 2007; 16: 377–383. [DOI] [PubMed] [Google Scholar]
- 37. Servaes P, Gielissen MF, Verhagen S, et al. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psycho-oncology 2007; 16: 787–795. [DOI] [PubMed] [Google Scholar]
- 38. Van Herck M, Spruit MA, Burtin C, et al. Fatigue is highly prevalent in patients with asthma and contributes to the burden of disease. J Clin Med 2018; 23: E471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 40. Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014; 44: 1504–1520. [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbation. Chest 2000; 117: 398s–401s. [DOI] [PubMed] [Google Scholar]
- 42. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93: 580–586. [DOI] [PubMed] [Google Scholar]
- 43. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 44. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532–555. [DOI] [PubMed] [Google Scholar]
- 45. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: NJ, Erlbaum, 1988. [Google Scholar]
- 46. Goertz YMJ, Looijmans M, Prins JB, et al. Fatigue in patients with chronic obstructive pulmonary disease: protocol of the Dutch multicentre, longitudinal, observational FAntasTIGUE study. BMJ Open 2018; 8: e021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reishtein JL. Relationship between symptoms and functional performance in COPD. Res Nurs Health 2005; 28: 39–47. [DOI] [PubMed] [Google Scholar]
- 48. Woo K. A pilot study to examine the relationships of dyspnoea, physical activity and fatigue in patients with chronic obstructive pulmonary disease. J Clin Nurs 2000; 9: 526–533. [DOI] [PubMed] [Google Scholar]
- 49. Kinsman RA, Yaroush RA, Fernandez E, et al. Symptoms and experiences in chronic bronchitis and emphysema. Chest 1983; 83: 755–761. [DOI] [PubMed] [Google Scholar]
- 50. Hanania NA, Mullerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med 2011; 183: 604–611. [DOI] [PubMed] [Google Scholar]
- 51. Biswas D, Mukherjee S, Chakroborty R, et al. Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J Clin Diagn Res 2017; 11: OC24–OC27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Economou N-T, Ilias I, Velentza L, et al. Sleepiness, fatigue, anxiety and depression in Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea – Overlap – Syndrome, before and after continuous positive airways pressure therapy. PLoS One 2018; 13: e0197342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vardar-Yagli N, Calik-Kutukcu E, Saglam M, et al. The relationship between fear of movement, pain and fatigue severity, dyspnea level and comorbidities in patients with chronic obstructive pulmonary disease. Disabil Rehabil 2018; 10: 1–5. [DOI] [PubMed] [Google Scholar]
- 54. Stridsman C, Müllerova H, Skär L, et al. Fatigue in COPD and the impact of respiratory symptoms and heart disease: a population based study. COPD 2013; 10: 12–132. [DOI] [PubMed] [Google Scholar]
- 55. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 728–735. [DOI] [PubMed] [Google Scholar]
- 56. Matura LA, Malone S, Jaime-Lara R, et al. A systematic review of biological mechanisms of fatigue in chronic illness. Biol Res Nurs 2018; 20: 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Al-Shair K, Kolsum U, Dockry R, et al. Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respir Res 2011; 12: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Larson R, Csikszentmihalyi M. The experience sampling method. In: Reis HT. (ed.) Naturalistic approaches to studying social interaction: new directions for naturalistic methods in the behavioral sciences. Vol. 15 San Francisco, CA: Jossey-Bass, 1983, pp.41–56. [Google Scholar]
- 59. Maes IH, Delespaul PA, Peters ML, et al. Measuring health-related quality of life by experiences: the experience sampling method. Value Health 2015; 18: 44–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Revision_Online_supplement_FatigueCOPD_Ther_Adv_Resp_Dis_YMJGoertz_3April2019 for Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation by Yvonne M. J. Goërtz, Martijn A. Spruit, Alex J. Van ‘t Hul, Jeannette B. Peters, Maarten Van Herck, Nienke Nakken, Remco S. Djamin, Chris Burtin, Melissa S. Y. Thong, Arnold Coors, Yvonne Meertens-Kerris, Emiel F. M. Wouters, Judith B. Prins, Frits M. E. Franssen, Jean W. M. Muris, Lowie E. G. W. Vanfleteren, Mirjam A. G. Sprangers, Daisy J. A. Janssen and Jan H. Vercoulen in Therapeutic Advances in Respiratory Disease