Abstract
Nasopharyngeal carcinoma (NPC) is an epithelial cancer of the nasopharynx which is highly associated with Epstein–Barr virus (EBV). Worldwide, most of the top 20 countries with the highest incidence and mortality rates of NPC are low‐ and middle‐income countries. Many studies had demonstrated that EBV could be detected in the tissue, serum and plasma of NPC patients. In this study, we explored the potential of assays based on non‐invasive nasal washings (NW) as a diagnostic and prognostic tool for NPC. A total of 128 patients were evaluated for NW EBV DNA loads and a subset of these samples were also tested for 27 EBV and human miRNAs shortlisted from literature. EBV DNA and seven miRNAs showed area under the receiver operating characteristic curve (AUC) values of more than 0.7, suggestive of their potential utility to detect NPC. Logistic regression analyses suggested that combination of two NW assays that test for EBNA‐1 and hsa‐miR‐21 had the best performance in detecting NPC. The trend of NW EBV DNA load matched with clinical outcome of 71.4% (10 out of 14) NPC patients being followed‐up. In summary, the non‐invasive NW testing panel may be particularly useful for NPC screening in remote areas where healthcare facilities and otolaryngologists are lacking, and may encourage frequent testing of individuals in the high risk groups who are reluctant to have their blood tested. However, further validation in an independent cohort is required to strengthen the utility of this testing panel as a non‐invasive detection tool for NPC.
Keywords: nasal washings, nasopharyngeal carcinoma, EBV DNA load, microRNAs
Short abstract
What's new?
Nasopharyngeal carcinoma (NPC) is highly associated with Epstein–Barr virus (EBV) and is mostly prevalent in Southeast Asia. While EBV serology and EBV DNA load from nasopharyngeal swab or plasma are valuable early detection tests, a lack of resources has limited their implementation. This study found that the performance of nasal washings EBV DNA testing for the detection of NPC is superior to urine EBV DNA testing but inferior to EBV DNA testing from nasopharyngeal swab and blood. Nonetheless, nasal washings testing may be valuable in remote areas lacking healthcare facilities and lower invasiveness may encourage frequent testing for high‐risk groups.
Abbreviations
- AUC
area under curve
- CI
confidence interval
- EBV
Epstein–Barr virus
- miRNA
microRNA
- NPC
nasopharyngeal carcinoma
- NW
nasal washings
- ROC
receiver operating characteristics
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial cancer of the nasopharynx. It is highly prevalent in Southeast Asia and the Southern part of China but very rare in most part of the world. Family members of NPC patients have four‐ to eight‐fold higher risk than the general population in developing NPC.1 According to GLOBOCAN's estimates, 17 of the 20 countries with highest NPC incidence and mortality rates worldwide are low‐ and middle‐income countries (LMICs).2 In Malaysia, the group with lowest social class were reported to have a four‐fold higher risk of disease3 and some of the high incidence regions are in remote areas where access to healthcare is limited. Majority of NPC cases frequently presented late4 leading to poor survival rate.5
The aetiology of NPC is a complicated interaction between genetic, dietary and viral (Epstein Barr Virus [EBV]) factors.6 Reports showed that EBV could be detected in NPC cells most of the time, while traces of EBV could also be found in the blood, urine and saliva of NPC patients.7, 8, 9 Early detection tests such as EBV serology, EBV DNA load from nasopharyngeal swab/brush and plasma are valuable for NPC screening and patient management. Recent large cohort NPC screening studies in Southern China had demonstrated the utilities of these tests as early detection tool for NPC.10, 11 However, these NPC screening tests have yet to be adopted in LMICs with high NPC incidence rates due to many reasons. Lack of resources to store and process biospecimen for screening tests as well as access to endoscopic examination by otolaryngologists are among some of the reasons. In LMICs and remote areas, a useful non‐invasive test may encourage frequent testing of individuals from the high risk groups for early detection and aids in the monitoring of disease recurrence.
MiRNAs are a group of small non‐coding RNAs which alter gene expression post‐transcriptionally12 and its dysregulation has been identified in many cancers including NPC.13, 14 Over the course of about a decade, miRNA signatures unique to NPC had been reported based on studies in tissue, serum and plasma. As discordance between tissue miRNAs and circulating miRNAs is a known issue15 and it is assumed that miRNAs in NW samples may be more similar to cellular miRNAs than circulating miRNAs, five published Gene Expression Omnibus (GEO) datasets were compared to identify miRNAs which were consistently dysregulated between NPC and control tissues. Together with EBV DNA loads, levels of selected EBV and human miRNAs were examined in nasal washings (NW) of NPC patients and non‐NPC patients. The aims of this study were (i) to evaluate the diagnostic value of NW EBV DNA and NW miRNAs for NPC and, (ii) to determine if NW EBV DNA could be utilised as a monitoring tool for post‐treatment NPC patients.
Materials and Methods
Patients and samples
Patients were recruited from the Department of Otorhinolaryngology in Selayang Hospital and the Department of Oncology and Radiotherapy in Kuala Lumpur Hospital with informed consent and ethics approval from the Medical Research and Ethics Committee, Ministry of Health Malaysia. All NPC patients were histologically diagnosed as NPC while controls were non‐NPC patients from the otorhinolaryngology clinic. Details of the non‐NPC group are depicted in Figure 1. Collection of NW sample was carried out by the patient himself/herself. Five millilitres of saline was pushed through the left nostril to the right nostril from a syringe and nasal washings was collected in a kidney dish. The same procedure was repeated for the right nostril to the left nostril. The combined nasal washings were then poured into a plastic bottle and stored at −20 °C.
Figure 1.
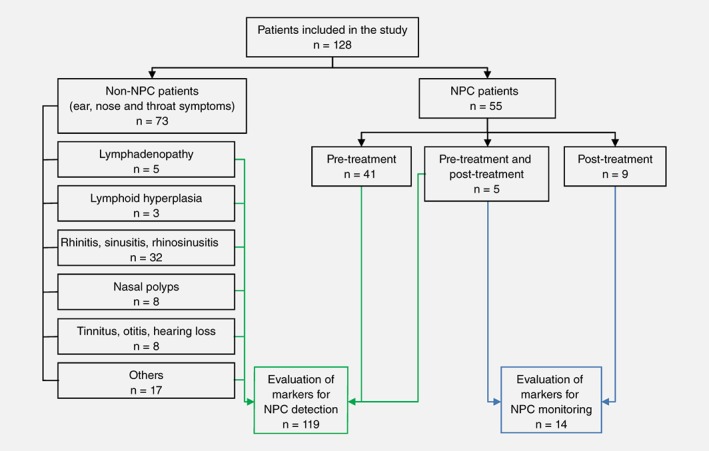
Overview of experimental design. EBV DNA and microRNAs from the nasal washings (NW) of non‐NPC and pre‐treatment NPC were evaluated to develop detection tool for NPC (indicated by solid line). Multiple time points NW samples from NPC patients were included in the evaluation of EBV DNA as a monitoring tool for NPC (indicated by dashed line). [Color figure can be viewed at wileyonlinelibrary.com]
DNA and RNA extractions
Frozen NW samples were thawed and centrifuged at 4000 RPM, 4 °C for 20 min. Cell pellets were resuspended with 400 μL of phosphate buffered saline and separated into two 200 μL aliquots for separate extraction of DNA and RNA. DNA and RNA extractions were performed using QIAamp DNA Mini kit and miRNeasy Micro Kit (Qiagen, Germany), respectively, according to manufacturer's instructions. Synthetic miRNAs (cel‐miR‐39 and cel‐miR‐54) were spiked in during RNA extraction after the addition of lysis buffer.
Quantification of EBV DNA
EBV DNA loads were evaluated by quantifying PCR products amplified from the BamHI‐W region and EBNA‐1 of EBV. Sequences of primers and probes used for the detection of EBV DNA were according to previous publications,7, 16 with FAM and MGB as fluorochrome and quencher, respectively for probes. Quantitative Polymerase Chain Reaction (qPCR) was carried out in triplicates for each sample. No‐template‐control and a series of standard points from serial dilution of Namalwa cells DNA were run in each qPCR plate. EBV DNA copy number was interpolated from the standard curve and results were calculated using the following formula:
(C q = quantitation cycle, c = intercept, m = slope of the standard curve)
All EBV DNA positive NW samples with C q value in only one qPCR wells and/or out of the interpolation range were arbitrarily set as one copy EBV DNA.
Evaluation of miRNA expression
Twenty‐seven miRNAs were shortlisted from GEO DataSets for further validation in RT‐qPCR (Supporting Information Table S1). Selection criteria is described in Supporting Information Methods.
RNA samples were subjected to reverse transcription (RT), preamplification and qPCR carried out in triplicates according to the optimised protocol that had demonstrated high consistency of miRNA detection in high‐throughput microfluidic platform.17, 18 No‐template‐control and a series of standard points from serial dilution of pooled human cell/xenograft RNA were run in the same dynamic array. Assays that did not exhibit linear amplification and data points that were beyond the reliable detection limit (C q > 25) were excluded from analysis. Average Cq values for NW were normalised to spiked‐in synthetic oligonucleotides to remove technical bias.17 For comparison of miRNA levels between NW samples, fold change over the detection limit was calculated using the following formula:
Statistical analysis
Mann Whitney test was applied to compare the differences between two groups of samples. Area under the curve (AUC) of receiver operating characteristic (ROC) were calculated to evaluate the performance of markers as classifier for NPC. Optimal cut‐off values were obtained by calculating Youden's index. Logistic regression was used to determine if combination of markers could lead to improved classifier performance for NPC.
Results and Discussions
Characteristics of study population
A total of 128 patients comprising of 55 NPC and 73 non‐NPC were assessed for their NW EBV DNA loads and miRNA levels. Samples collected at multiple time points from 14 NPC patients and their follow‐up information were available for analysis. Overview of the experimental details is shown in Figure 1. Between the non‐NPC patient samples and pre‐treatment NPC samples, there were no significant differences in the distribution of age, ethnicity and gender (Supporting Information Table S2).
NW EBV DNA load as biomarker for NPC detection and monitoring
In our study, we showed that even though NW sampling was carried out by different operators (the test subject himself/herself), NW EBV DNA test results were consistent and reproducible (Supporting Information Fig. S1). Pre‐treatment NPC samples were shown to have significantly higher NW EBV DNA load than the non‐NPC samples (Figs. 2 a and 2b). ROC analysis suggested that NW EBV DNA is good at classifying non‐NPC from pre‐treatment NPC patients (Fig. 2 j and Supporting Information Table S3). Patients with larger tumour size (T3 and T4) appeared to have higher median of NW EBV DNA load as compared to those with smaller tumour size (T1 and T2) (Supporting Information Fig. S2). When remission samples were compared to residual NPC samples, higher median NW EBV DNA load was seen in the residual NPC samples (Supporting Information Fig. S3).
Figure 2.
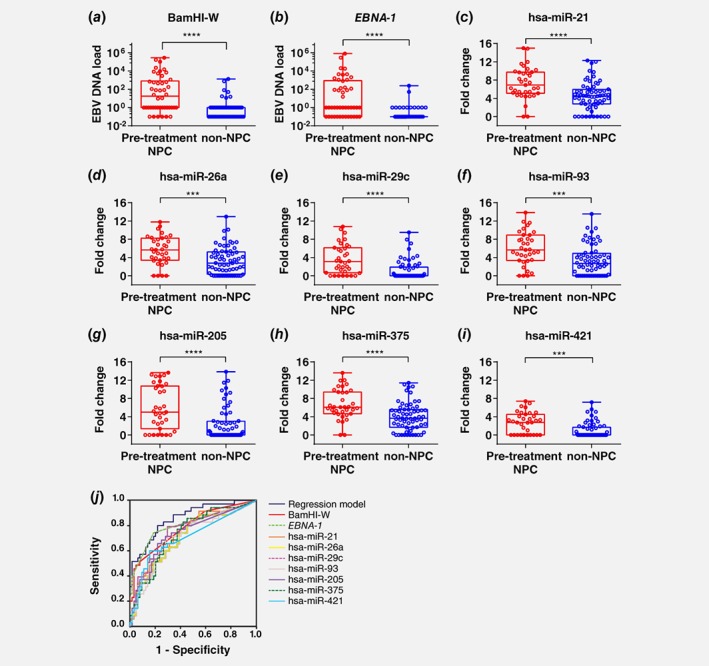
EBV DNA load and differentially expressed miRNAs in nasal washings. EBV DNA load measured by (a) BamHI‐W and (b) EBNA‐1 were significantly different between pre‐treatment NPC samples (n = 46) and non‐NPC samples (n = 73) (Mann–Whitney test, **** p ≤ 0.0001). EBV DNA load was set as 0.1 (arbitrary value) for samples below detection limit (Cq > 40) and 1 to indicate samples that were weak positive. (c) hsa‐miR‐21, (d) hsa‐miR‐26b, (e) hsa‐miR‐29c, (f) hsa‐miR‐93, (g) hsa‐miR‐205, (h) hsa‐miR‐375 and (i) hsa‐miR‐421 in nasal washings of pre‐treatment NPC (n = 35) and non‐NPC (n = 64) samples were significantly different (Mann Whitney test, *** p ≤ 0.001; **** p ≤ 0.0001). (j) Receiver operating characteristics curve discriminating pre‐treatment NPC from non‐NPC. Area under curve for all markers are above 0.7 as shown in Supporting Information Table S4. [Color figure can be viewed at wileyonlinelibrary.com]
Different NW EBV DNA trends were observed in NW samples collected at multiple time points from 14 NPC patients (Fig. 3). Among these cases, 71.4% (10/14, Figs. 3 a–3 j) had NW EBV DNA trends that corresponded to their clinical outcomes. Consistently undetectable or decreasing NW EBV DNA load was observed in eight of the remission cases (Figs. 3 a–3 h), while increasing NW EBV DNA load was observed in a recurrent NPC (Fig. 3 i) and a residual NPC (Fig. 3 j). For the case depicted in Figure 3 i, follow‐up clinical examination and cytology test of nasal swab reported no malignancy but increasing NW EBV DNA load was detected. This case was not clinically diagnosed as recurrence until 10 months later. For the case shown in Figure 3 j, consistent high levels of NW EBV DNA over two post‐treatment sampling time points corresponded well with the clinical diagnosis of residual NPC. Among the four cases which NW EBV DNA load did not correspond to the clinical outcome, possible reasons include: (i) false positive, as the increment was from undetected to one copy (Fig. 3 k), (ii) no tumour in the nasopharynx leading to negative NW EBV DNA test results even though patients had distant metastasis (Figs. 3 l and 3m) and (iii) possible true EBV negative for recurrent NPC (Fig. 3 n). Limitation of NW EBV DNA test to detect EBV negative NPC cases suggested the importance to include non‐EBV markers in the detection panel.
Figure 3.
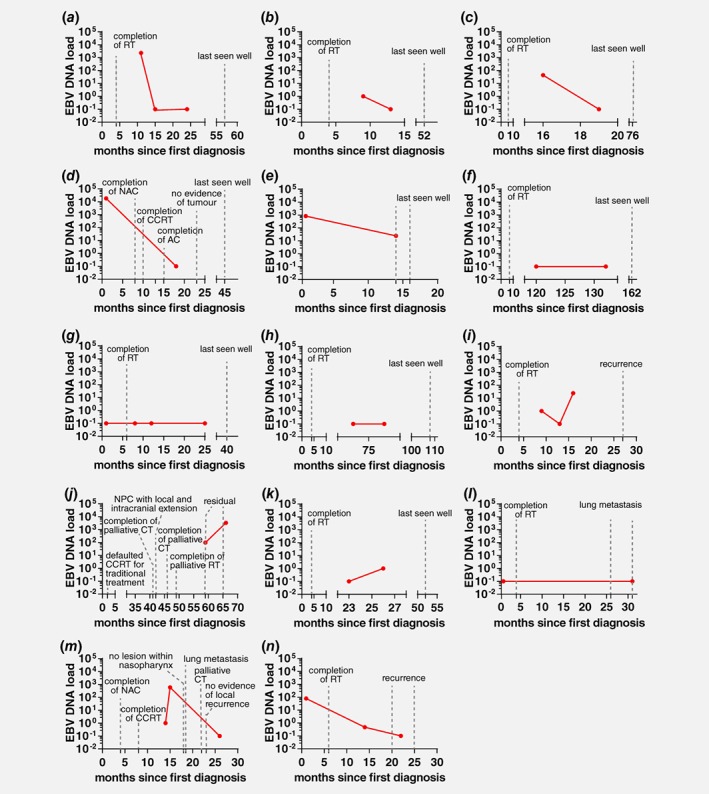
EBV DNA load in nasal washings of NPC patients collected at multiple time points. Each graph represents the trend of NW EBV DNA load (measured by EBNA‐1 assay) for one patient. Each dot represents NW EBV DNA load at the indicated time point. (a)–(h) Patients were last known to be well and had decreasing or undetectable NW EBV DNA load after treatment. (i) and (j) Increasing NW EBV DNA load in patients with recurrence/residual NPC. (k) Undetectable and weak positive NW EBV DNA load in complete remission patient. (l)–(n) Undetectable or decreasing NW EBV DNA load in patients with lung metastasis or recurrence. RT, radiotherapy; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy; CCRT, concurrent chemo‐radiotherapy; CT, chemotherapy. [Color figure can be viewed at wileyonlinelibrary.com]
NW miRNA as biomarker for NPC detection
GEO2R analysis on five NPC tissue miRNA profiling data sets obtained from NCBI GEO database (GSE70790, GSE43039, GSE32960, GSE36682 and GSE32906) was performed to identify dysregulated miRNAs in NPC. Thirteen consistently dysregulated miRNAs in at least three of the five studies, together with an additional 14 miRNAs dysregulated in at least one of the five studies were selected for further validation (Supporting Information Table S1).
Due to insufficient material, only 35 NPC and 64 non‐NPC samples were evaluated for NW miRNAs. One out of 27 miRNA assays failed and was excluded from further analysis (Supporting Information Table S1). Among the miRNAs analysed, 69.3% (18/26) were detected in NW samples. Low and inconsistent levels of RNU6B, RNU44 and RNU48 in NW samples (data not shown) suggested that there were limited cellular RNA and a mixture of cell‐free miRNAs from biofluid within the nasal cavity. As a result, these endogenous small RNA were not applicable as reference gene for NW data normalisation. Based on data normalised to cel‐miR‐39 spike‐in controls, RT‐qPCR results indicated that 38.9% (7/18) of the miRNAs detected in NW, namely hsa‐miR‐21, hsa‐miR‐26a, hsa‐miR‐29c, hsa‐miR‐93, hsa‐miR‐205, hsa‐miR‐375 and hsa‐miR‐421 were significantly upregulated in pre‐treatment NPC compared to non‐NPC NW samples (Figs. 2 c‐2i). In ROC analysis, the AUC of these miRNAs were all above 0.7 in classifying non‐NPC from pre‐treatment NPC (Fig. 2 j and Supporting Information Table S3). Levels of hsa‐miR‐21, hsa‐miR‐26a, hsa‐miR‐29c and hsa‐miR‐93 appeared to be associated with larger tumour size (Supporting Information Fig. S2).
Two of the upregulated NW miRNAs, hsa‐miR‐93 and hsa‐miR‐205 concurred with the findings of other studies.19, 20 Both hsa‐miR‐93 and hsa‐miR‐205 were shown to be upregulated in NPC tissue samples21, 22 and could lead to enhance cell growth, migration and invasion in NPC cell lines.21, 23 Meanwhile, hsa‐miR‐21 and hsa‐miR‐421 that were shown to be upregulated in NW were consistent with the trend reported by 1 to 2 NPC tissue profiling studies (Supporting Information Table S1). Three of the upregulated NW miRNAs, namely hsa‐miR‐26a, hsa‐miR‐29c and hsa‐miR‐375, were previously reported to be downregulated in NPC tissues as compared to control tissues.20, 24, 25 Discordance of circulating miRNA profiles with tissue miRNA profiles is a common issue.15 Studies of NPC tissues compare cellular miRNAs between tumour and normal epithelial cells while our study using NW analysed a mixture of cellular and cell‐free miRNAs from tumour, as well as from other cell types and biofluid within the nasal cavity. Hence differences in levels may also not be directly comparable. Nonetheless, we postulate that, just like EBV DNA, the significantly upregulated NW miRNAs which were associated with tumour size are highly enriched in tumour cells.
Performance of combined markers for NPC detection
NW EBV DNA and seven microRNAs that were shown to have significant differences between the pre‐treatment NPC and non‐NPC, as well as AUC > 0.7 were included in the logistic regression analyses. Simple logistic regression demonstrated that all nine markers (BamHI‐W, EBNA‐1, hsa‐miR‐21, hsa‐miR‐26a, hsa‐miR‐29c, hsa‐miR‐93, hsa‐miR‐205, hsa‐miR‐375 and hsa‐miR‐421) were able to predict NPC with p < 0.05 (Supporting Information Table S4). In multiple logistic regression analyses using forward and backward methods to evaluate all markers shortlisted from simple logistic regression analyses, only EBNA‐1 and hsa‐miR‐21 remained as the significant variables in the model. Multicollinearity was detected and moderate correlation was observed between BamHI‐W and EBNA‐1, as well as among few miRNAs, namely hsa‐miR‐21, hsa‐miR‐26a, hsa‐miR‐29c, hsa‐miR‐93 and hsa‐miR‐205. This could have led to the exclusion of these markers in the model for the prediction of NPC. Multiple logistic regression model with EBNA‐1 and hsa‐miR‐21 showed the best AUC (0.860) compared to any marker alone as a prediction model (Supporting Information Table S3). The utility of this model needs to be evaluated further by performing validation in an independent cohort in the future which should also include samples from non‐NPC patients with other EBV related diseases and EBV negative NPC patients.
Comparison of NW test to other less/minimally invasive test
Besides NW samples, EBV DNA test can be carried out using blood and other less/minimally invasive sample types, including urine and nasopharyngeal swab/brushings (Supporting Information Table S5). Among these sample types, otolaryngologist is required to collect nasopharyngeal swab/brushings samples, medical support staff would be needed for blood collection while collection of urine and NW samples could be done by the individual with guidance from the medical support personnel. The cost for NW sampling is lower compared to blood and nasopharyngeal swab/brushings samples that require additional laboratory resources like centrifuge, preservation buffer and/or storage in ultra‐low temperature (−80°C). In terms of test performance as evaluated by sensitivity, specificity, positive predictive value and negative predictive value, NW EBV DNA test (EBNA‐1) is slightly inferior to nasopharyngeal swab/brushings EBV DNA test but outperformed the urine EBV DNA test7 (Supporting Information Table S5), probably due to the proximity of sampling at the nasopharyngeal area.
Conclusion
In summary, the performance of NW EBV DNA test for the detection of NPC is superior to urine EBV DNA test but inferior to EBV DNA test from nasopharyngeal swab/brushings and blood. In remote areas of LMICs with high prevalence of NPC, access to healthcare facilities and routine monitoring by otolaryngologists are limited. The advantage of NW EBV DNA test compared to nasopharyngeal swab/brushings is that sampling can be easily achieved without the presence of otolaryngologist. In addition, high risks groups and post‐treatment NPC patients may feel more inclined to be tested frequently as compared to blood test because sampling of NW is non‐invasive. In fact, nasal washing is a procedure routinely taught by the otolaryngologists to the post‐treatment NPC patients for maintenance of nasopharynx. NW test may have potential as a post‐treatment surveillance system where patients with positive NW results can be closely monitored for any sign of recurrence. Lastly, our preliminary findings suggest that NW EBV DNA, hsa‐miR‐21, hsa‐miR‐26a, hsa‐miR‐29c, hsa‐miR‐93, hsa‐miR‐205, hsa‐miR‐375 and hsa‐miR‐421 are potential markers for NPC detection. External validation is required to confirm their applicability as detection tool for NPC.
Supporting information
Supporting information
Acknowledgements
We thank the Director General of Health Malaysia for his approval of the publication of this manuscript. We also thank the Director of Institute for Medical Research Malaysia for her support of this study. We are grateful to all staff from the Department of Otorhinolaryngology in Selayang Hospital, Department of Oncology and Radiotherapy in Kuala Lumpur Hospital, Molecular Pathology unit and Biospecimen Bank at the Institute for Medical Research for their assistance in sample and data collection. This study was funded by Ministry of Health Malaysia (NMRR‐11‐597‐9667).
Conflict of interest: This study has no conflict of interest.
Contributor Information
Ching‐Ching Ng, Email: ccng@um.edu.my.
Lu Ping Tan, Email: luping@imr.gov.my.
References
- 1. Ng WT, Choi CW, Lee MCH, et al. Familial nasopharyngeal carcinoma in Hong Kong: epidemiology and implication in screening. Fam Cancer 2009;8:103–8. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D., Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. [cited 2018 Jul 29];Available from: http://globocan.iarc.fr
- 3. Armstrong RW, Imrey PB, Lye MS, et al. Nasopharyngeal carcinoma in Malaysian Chinese: salted fish and other dietary exposures. Int J Cancer 1998;77:228–35. [DOI] [PubMed] [Google Scholar]
- 4. Pua KC, ASB K, Yap YY, et al. Nasopharyngeal carcinoma database. Med J Malaysia 2008;63(Suppl C):59–62. [PubMed] [Google Scholar]
- 5. Lee AWM, Ng WT, Chan YH, et al. The battle against nasopharyngeal cancer. Radiother Oncol 2012;104:272–8. [DOI] [PubMed] [Google Scholar]
- 6. Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol 2012;22:233–44. [DOI] [PubMed] [Google Scholar]
- 7. Chan KCA, Leung SF, Yeung SW, et al. Quantitative analysis of the transrenal excretion of circulating EBV DNA in nasopharyngeal carcinoma patients. Clin Cancer Res 2008;14:4809–13. [DOI] [PubMed] [Google Scholar]
- 8. Ji M‐F, Huang Q‐H, Yu X, et al. Evaluation of plasma Epstein‐Barr virus DNA load to distinguish nasopharyngeal carcinoma patients from healthy high‐risk populations in southern China. Cancer 2014;120:1353–60. [DOI] [PubMed] [Google Scholar]
- 9. Pow EHN, Law MYT, Tsang PCS, et al. Salivary Epstein‐Barr virus DNA level in patients with nasopharyngeal carcinoma following radiotherapy. Oral Oncol 2011;47:879–82. [DOI] [PubMed] [Google Scholar]
- 10. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein–Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 2017;377:513–22. [DOI] [PubMed] [Google Scholar]
- 11. Liu Z, Ji MF, Huang QH, et al. Two Epstein‐Barr virus‐related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in southern China. Am J Epidemiol 2013;177:242–50. [DOI] [PubMed] [Google Scholar]
- 12. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 13. Bruce JP, Hui ABY, Shi W, et al. Identification of a microRNA signature associated with risk of distant metastasis in nasopharyngeal carcinoma. Oncotarget 2015;6:4537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cai L, Ye Y, Jiang Q, et al. Epstein‐Barr virus‐encoded microRNA BART1 induces tumour metastasis by regulating PTEN‐dependent pathways in nasopharyngeal carcinoma. Nat Commun 2015;6:7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jarry J, Schadendorf D, Greenwood C, et al. The validity of circulating microRNAs in oncology: five years of challenges and contradictions. Mol Oncol 2014;8:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevens SJC, Verkuijlen SAWM, Hariwiyanto B, et al. Diagnostic value of measuring Epstein‐Barr virus (EBV) DNA load and carcinoma‐specific viral mRNA in relation to anti‐EBV immunoglobulin a (IgA) and IgG antibody levels in blood of nasopharyngeal carcinoma patients from Indonesia. J Clin Microbiol 2005;43:3066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan GW, Khoo ASB, Tan LP. Evaluation of extraction kits and RT‐qPCR systems adapted to high‐throughput platform for circulating miRNAs. Sci Rep 2015;5:9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan GW, Tan LP. High‐throughput RT‐qPCR for the analysis of circulating MicroRNAs In: Dalmay T, ed. MicroRNA detection and target identification: methods and protocols. New York, NY: Springer New York, 2017. 7–19. [DOI] [PubMed] [Google Scholar]
- 19. Luo Z, Zhang L, Li Z, et al. An in silico analysis of dynamic changes in microRNA expression profiles in stepwise development of nasopharyngeal carcinoma. BMC Med Genomics 2012;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu N, Chen NY, Cui RX, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol 2012;13:633–41. [DOI] [PubMed] [Google Scholar]
- 21. Xu YF, Mao YP, Li YQ, et al. MicroRNA‐93 promotes cell growth and invasion in nasopharyngeal carcinoma by targeting disabled homolog‐2. Cancer Lett 2015;363:146–55. [DOI] [PubMed] [Google Scholar]
- 22. Tang J‐F, Yu Z‐H, Liu T, et al. Five miRNAs as novel diagnostic biomarker candidates for primary nasopharyngeal carcinoma. Asian Pac J Cancer Prev 2014;15:7575–81. [DOI] [PubMed] [Google Scholar]
- 23. Mao Y, Wu S, Zhao R, et al. MiR‐205 promotes proliferation, migration and invasion of nasopharyngeal carcinoma cells by activation of AKT signalling. J Int Med Res 2016;44:231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sengupta S, den Boon JA, Chen I‐H, et al. MicroRNA 29c is down‐regulated in nasopharyngeal carcinomas, up‐regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA 2008;105:5874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hui ABY, Bruce JP, Alajez NM, et al. Significance of dysregulated metadherin and microRNA‐375 in head and neck cancer. Clin Cancer Res 2011;17:7539–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


